Acylation is an organic chemical process in which an acyl group is added to a molecule. An acyl group is a functional group having the chemical formula R-C=O.
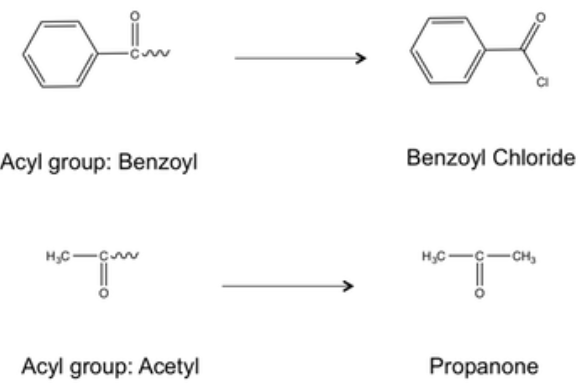
Friedel and Crafts reported the synthesis of an aryl ketone using carboxylic acid chloride, aluminium chloride, and benzene in 1877. Carboxylic acids, as well as carboxylic acid derivatives, esters, and anhydrides, can now be used in Friedel-Crafts acylation. These electrophilic aromatic substitutions are also promoted by a wide range of Lewis and Bronsted acids. The Friedel-Crafts acylation is a critical industrial conversion since it is utilised to prepare chemical feedstock, synthetic intermediates, and speciality compounds.
Despite the long history of Friedel-Crafts acylation, hardly any reports of amides being utilised in these conversions have been published. The majority of Friedel-Crafts acylations are considered to take place via reactive acyl cation intermediates. Under acidic circumstances, however, the strong carbon-nitrogen bond in amides prevents cleavage to acyl cations. As a result, amides are no longer employed in Friedel-Crafts acylation. Though amides are not typically thought to be suitable substrates for the Friedel-Crafts synthesis of aromatic ketones, new research has demonstrated that destabilised amides may produce these compounds in high yields. Friedel-Crafts reactions, for instance, have been demonstrated to produce aryl ketones from β-lactams.
What Is the Acylation Reaction of Friedel-Crafts?
A popular acylation process is the Friedel-Crafts acylation. The reaction’s name was obtained from the scientists who discovered it, Charles Friedel and James Crafts.
The distinction between mechanism and reaction would be Friedel-Crafts acylation. A mechanism of electrophilic aromatic substitution would be employed to carry out this reaction. This mechanism is used to add acyl groups to aromatic compounds via the Friedel-Crafts acylation process.
Ethanol chloride is a typical acylating substance used in Friedel-Crafts acylations. The most common catalyst is an acyl halide, such as aluminium chloride.
Here’s a simplified diagram of the Friedel-Crafts acylation of benzene.
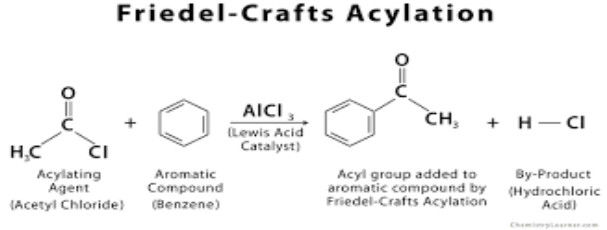
The method of Benzene Acylation in Friedel-Crafts
- The Lewis acid, such as an atom, ion, or molecule with an incomplete octet of electrons, can act as a Lewis acid (e.g., BF3, AlF3) combines with the acyl halide to produce a complex.
- The electrophilic acylium ion is formed when the halide reacts with the Lewis acid, such as an atom, ion, or molecule with an incomplete octet of electrons.
- The aromatic electrons work as nucleophiles, attacking the electrophilic C+. This process eliminates the aromaticity, resulting in the cyclohexadienyl cation intermediate.
Removing the proton from the acyl-group-containing sp3 C restores the C = C and aromatic systems, creating HCl and restoring the active catalyst.
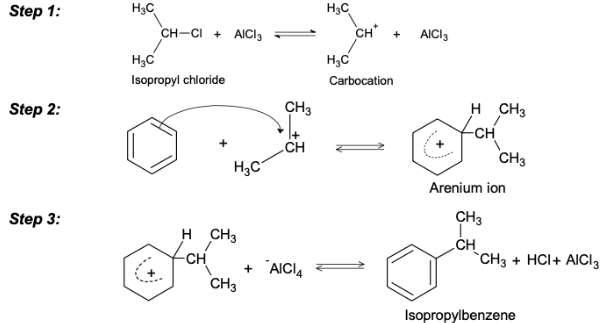
Limitations of Friedel-Crafts Alkylation
Carbocation rearrangements are possible while attempting to add a carbon chain with more than two carbons. The rearrangements are caused by hydride and methyl shifts. When a three-carbon chain is added as a substituent, the result of a Friedel-Crafts Alkylation will exhibit an iso rearrangement. Friedel-Crafts Acylation is one method for resolving these issues.
Furthermore, the reaction will only take place if the ring to which you are adding a substituent is not deactivated. Friedel-Crafts fail when employed with chemicals such as nitrobenzene and other strong deactivating systems.
Friedel-Crafts processes cannot be accomplished when the aromatic ring has an NH2, NHR, or NR2 substituent. The amines’ lone pair electrons react with the Lewis acid AlCl3. This adds a positive charge to the benzene ring, which is so powerful that the Friedel-Crafts reaction is prevented.
Finally, Friedel-Crafts alkylation can be poly alkylated. The process introduces an electron-donating alkyl group, which activates the benzene ring and allows it to be further alkylated.
Because an acyl group is deactivating, this issue does not occur during Friedel-Crafts acylation. This inhibits subsequent acylations.
The Importance of Acylation
The acylation reaction is an important step in both biological and chemical processes. This process is used in actions that are essential to numerous biological activities, such as protein production and control. In chemistry, you may come across the acylation method being used to create industrial chemicals for specialised applications, such as the fabrication of plastic items. Acylation is also used in the production of pharmaceuticals.
Conclusion
In terms of synthetic efficiency, Friedel-Crafts Acylations outperform Friedel-Crafts Alkylations. These advantages provide you with greater control over the formation of reaction products. There is no carbocation rearrangement because the acylium ion is stabilised by resonance. Furthermore, for EAS reactions, acyl groups deactivate, so the result does not conduct further reactions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





