When any reaction takes place, products are formed by the reactants. A reaction takes place under many circumstances and conditions which may or may not be necessary to carry out a particular reaction. What is the basic property of these reactions that start them? These reactions are started by activation energy. It is the minimum amount of energy required by reactants to start the reaction. It is input energy that is required to start the reaction. Here are notes and some activation energy formula questions.
Activation Energy: Overview
The activation energy formula can also be called the Arrhenius equation. The Arrhenius formula works under limited conditions only. For example, the proteins show many folding and unfolding transitions. In such cases, the problem is shown because of the range of temperature, which is limited.
Many other terms are related to the activation energy and activation energy formula, such as rate constant, Gibbs energy, diffusion constants, transport coefficients. Activation energy has many applications in quantum dynamics too.
Activation Energy is the minimum energy that the reactants must have so that the required reaction can start and take place. The Activation Energy Formula can be derived as the Arrhenius equation. The Activation Energy Formula is K = Ae− EaRT
A simple example of activation energy is that a car needs some energy to start. When you turn the key on, it activates the burning of the petrol which offers the energy to start a car.
The Activation Energy Formula is calculated in joules per mole. The activation energy was introduced in the year 1889. It was introduced by Svante Arrhenius, the name on which the Activation Energy Formula was given later. Activation energy and Activation Energy Formula are widely used in the applications of nuclear reactions. And numerous other phenomena of physics. Activation energy does require. Enzymes in our bodies also act as activation energy. Also, to reach the transition energy, activation energy plays a very important role.
Activation Energy Formula Notes
The Activation Energy formula certainly is
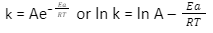
Here, A turns out to be the pre exponential factor. Moreover, the reaction is almost constant. In addition, the reaction relies on the temperature. Ea turns out to be the Activation Energy while the gas constant is R.
Additionally, T alludes to the temperature while k alludes to the reaction rate constant. Most importantly, the principle factor of a particular reaction is significant. The activation energy leads to the beginning of the reaction and moves it in a particular direction.
The activation energy formula determines numerous forces in the active states like van der Waals forces, hydrogen bonding, etc. However, the reactions which do not have a catalyst need higher activation energy. So, the activation energy formula is used to determine this energy also.
The activation energy formula determines numerous forces in the active states like van der Waals forces, hydrogen bonding, etc. However, the reactions which do not have a catalyst need higher activation energy. So, the activation energy formula is used to determine this energy also.
The activation energy formula is different for one-step reactions and chain reactions. The activation energy formula also affects the Gibbs energy. It tells whether Gibbs’s energy is positive or negative. The all-over change in energy does not depend on the activation energy. Also, activation energy does not determine if the reaction is spontaneous.
Sometimes an increase in temperature results in the reduction of the rate of reaction. This results in negative activation energy through the activation energy formula.
Factors Affecting Activation Energy
In the activation energy formula,k stands for the rate constant, A stands for factor, which is pre-exponential, e stands for activation energy, R is the universal gas constant, and T is the absolute temperature in kelvin.
Many factors affect activation energy.
Nature of Reactants
The value of activation energy will be low in the case of ionic reactants. This is because they have an attraction between the species of reactants. Now, in the case of covalent reacting species, the activation energy will be high. This is because some energy is needed to break their bonds.
Catalyst Effect
The activation energy will be low if the reaction has a positive catalyst. This is because a catalyst provides a better way to complete the reaction. In the case of a negative catalyst, the activation energy will be high.
The activation energy formula or activation energy does not depend upon the factors like volume, temperature, pressure, reactant coefficient, etc.
Conclusion
The activation energy formula is widely used to determine the energy needed by the reactant so that the reaction can start. Activation energy is the amount of energy that is minimally required at the reactant species to start the reaction. You must be thinking that the activation energy is different in different conditions like pressure, etc. But this is not true. Factors such as temperatures, pressures, volumes, etc., do not affect the activation energy.
There are many simpler examples of activation energy in our day-to-day lives. For example, starting a car, starting a laptop, starting a fire, etc.
The way these things energy to start, likewise chemical reactions also need an activation energy to begin. Endothermic reactions need a constant input of energy to keep going on. Exothermic reactions also need activation energy. Also, activation energy is needed so that the reactants can overcome repulsive forces. So that they can move the reaction further ahead and start making and breaking bonds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





