Activation Energy Examples
Assume you are hiking and need to climb a hill to get to the other side. You will need to give some effort to go up the slope. The higher the slope, the more energy you must use to get to the other side.
Molecules in biological processes also require energy to initiate a reaction. Molecules, for example, require some kinetic energy, or velocity, to collide with other molecules and trigger a reaction. There will be no reaction if the impacts do not occur frequently or have insufficient kinetic energy.
The activation energy is the amount of energy necessary to initiate a reaction. Just like the lower the slope you are trekking, the faster you get to the other side, in the same way, the smaller the activation energy, the quicker the reaction.
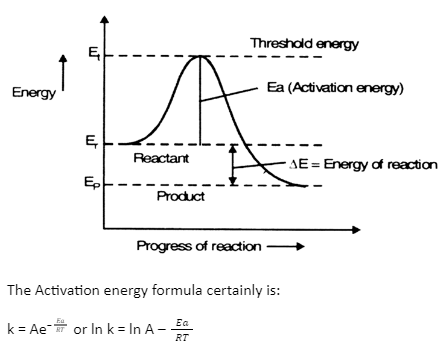
The energy of reactants and products are distinct. To be transformed into products, the reactants would have to travel through a transition state, generally greater in energy.
The system requires activation energy to reach this transition state. Finally, to reach the final product state, the products minimise their energy.
The energy of reactants is greater than products. The energy of reactants increases and eventually decreases to the end product’s energy. The highest point represents the energy of the intermediate state. The activation energy is the amount of energy to reach the intermediate state.
In Chemistry, How Does Activation Energy Work?
Chemical processes require a certain amount of energy to begin. The activation energy is the smallest energy necessary to initiate a reaction.
To comprehend activation energy, we must first consider how a chemical reaction occurs.
Anyone who has ever lit fire has an instinctive awareness of the process, even if they have not linked it to chemistry.
Many of us have a sense of the broad heat required to start a flame. We know that a single match will not suffice to light a massive wood, and a flame thrower would be overkill.
We also know that moist or thick materials demand more heat than dry materials. The activation energy is represented by the insufficient quantity of energy we know we need to start a fire.
Existing connections must be broken and new ones formed for a reaction to occur.
The reaction proceeds only if the products are more stable than the reactants. For example, in a fire, we transform carbon in wood into CO2, which is a more stable form of carbon than wood.
Therefore, the reaction continues and creates heat in the process. The activation energy, in this case, is the initial heat necessary to ignite the fire. Our efforts and matches are evidence of this.
In a chemical process, activation energy may be the barrier between the reactants and products’ minima (the minimum required values).
Activated complex examples
Creating a molecule such as a hydrogen iodide helps better comprehend the activated complex’s concept in the transition state. Assume a hydrogen atom comes into contact with halogen molecules to produce an active compound.
When both are separated by a significant distance, the system’s energy equals the sum of individual energies. The orbitals of the hydrogen and iodine atoms begin to overlap as the hydrogen atom approaches the iodine molecule.
As a result, the iodine-iodine chemical link begins to lengthen. The system’s energy is now being activated or increased due to the complex’s development.
Activation Energy formula
Here, A turns out to be the pre-outstanding element that works with the reaction. Moreover, the reaction is almost constant. In addition, the reaction relies on the temperature. Ea turns out to be the Activation Energy while the gas constant is R.
Additionally, T alludes to the temperature while k alludes to the reaction rate constant. Most importantly, one can note three significant variables whose fuse occurred by Arrhenius.
The principal factor is that the particles comprise potential, which is significant for the reaction. Furthermore, impacts happen between particles. Therefore, at long last, the quantity of impacts will generally have the element of suitable direction.
Where Can I Find Activation Energy?
To calculate activation energy, use the Arrhenius equation. Rewriting the Arrhenius equation and noting the change in reaction rate as temperature changes are one method:
log K = log A – Ea/2.303 RT
log (k2/k1) = Ea/2.303 R x (1/T1 − 1/T2)
For instance, as the temperature rises from 310 K to 330 K, the rate constant of a first-order reaction increases from 3×10-2 to 8 x 10-2. Determine the activation energy (Ea):
log (8 × 10 – 2/3 × 10 – 2) = Ea/2.303 R (1/310 – 1/330)
log 2.66 = Ea/2.303 R (1.95503 x 10 – 4)
0.4249 Ea/2.303 × 8.314 x (1.95503 x 10 – 4)
0.4249 = Ea/19.147 x (1.95503 x 10 – 4)
0.4249 = 1.02106 x 10 – 5 x Ea
Ea = 41613.62 J/mol or 41.614 kJ/mol
You may calculate activation energy by graphing ln k (natural logarithm of the rate constant) vs 1/T and using the slope of the resultant line:
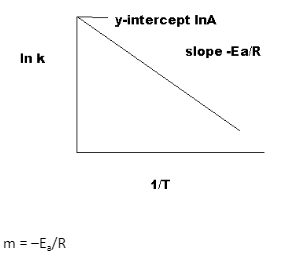
Here, m represents the slope of the line, Ea represents the activation energy, and R represents the ideal gas constant of 8.314 J/mol.K. Before calculating 1/T and plotting the graph, remember to convert any temperature readings acquired in Celsius or Fahrenheit to Kelvin.
Conclusion
Activation energy is commonly represented as Ea, which may be calculated using the Arrhenius mathematical formula, k = Ae – Ea/RT, where A = constant. It is self-evident that the Ea in the Arrhenius equation must contain energy units. It is commonly measured in joules per mole (J/mol), kilojoules per mole (kJ/mol), or kilocalories per mole (kcal/mol).
In an enzyme catalysis process in biology, the chemical catalyst can reduce the activation energy, allowing for more reaction in a given period through the production of the intermediate activated complex.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




