Activation energy is a notion in chemistry that was proposed in 1889 by a Swedish scientist named “Svante Arrhenius.” The lowest quantity of energy needed by chemical reactants to activate a chemical reaction is referred to as activation energy. The activation energy is sometimes described as the smallest amount of energy necessary to start a chemical reaction.
Have you ever wondered why a few chemical reactions are easy to perform, whereas others are in need of the addition of heat? When we combine metallic sodium with water, a fast reaction occurs, producing a tremendous amount of heat (needed enough to ignite the hydrogen gas that is formed). Group II metals, such as calcium, react at a very slow rate. In contrast to the extremely violent reaction with sodium, the reaction with calcium is gradual enough that the created hydrogen gas may be contained.
When reactant particles are given energy, the bond among the atoms vibrates at a higher frequency. When those particles clash with other particles, the hike in the energy of vibration makes it possible for a chemical bond to break and a chemical reaction to occur. The activation energy of a reaction is the least amount of energy required for interacting particles to go under a reaction. Several reactions happen easily at a decent temperature because the reacting particles most likely are supposed to have the all-important activation energy. Certain reactions happen only when heated since the particles don’t even have enough energy until an external source of heat supplies extra kinetic energy to the particles.
Activation energy definition
What defines activation energy is the sign “Ea”. Activation energy is denoted by the unit kL/mol or kcal/mol. According to Svante Arrhenius, the activation energy equation is:
K = A x e– Ea / RT
K is the rate of response coefficient.
A denotes the frequency factor.
The universal gas constant is R.
T is the Kelvin temperature.
Ea stands for activation energy.
The activation energy in transition state theory is narrated as the variation between the molecules or atoms of the given chemical reactant for the transition state in the transition state and those of the chemical reactants in the beginning state.
Significance of activation energy
When two chemicals are mixed, just a limited number of collisions between the reactant molecules occur spontaneously to produce products. This is especially true when the molecules have a low kinetic energy. As a result, before a sufficient percentage of reactants can be transformed into products, the system’s free energy must be overcome. The activation energy provides the reaction with the additional push it needs to get started.
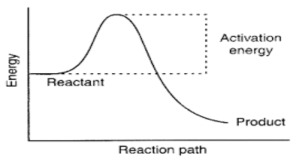
Exothermic processes, too, require activation energy to begin. A stack of logs, for instance, will not start burning on its own. A lighted match can supply the activation energy needed to initiate the burning process. When a chemical reaction begins, the heat produced by the reaction provides the activation energy required to transform the additional reactant into the product.
A scientific chemical reaction can take place without the addition of any additional energy. The activation energy of the reaction is frequently supplied by heat from the surrounding environment in this case. Heat makes the reactant molecules to travel faster, increasing the likelihood and force of collisions. The combination enhances the possibility that reactant bonds will break, allowing products to form.
Conclusion
Numerous reactions have such large activation energies that they do not occur at all in the absence of energy input. For example, while the burning of a fuel such as propane produces energy, the rate of reaction is virtually zero at ambient temperature. When a spark provides enough energy to push some molecules beyond the activation energy barrier, those molecules complete the reaction and release energy. The released energy assists additional fuel molecules in breaking through the energy barrier, resulting in a chain reaction.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





