The word acid is derived from ‘acidus’, which means sour. This is because of the characteristic of acids to produce a sour taste when added to water. An acid is a substance that can donate protons or hydrogen ions and accepts electrons in return. Acids have a hydrogen atom bonded to them that can dissociate to produce a cation and anion in water. Depending upon how concentrated the ions (hydrogen ions) are that an acid produces, the degree of its acidic nature is determined, and the level of pH solution is ascertained.
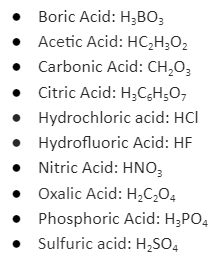
Dissolving an acid in the water produces a hydrogen ion and a nonmetal anion. The prefix hydro is added before the nonmetal’s name, and the ‘ide’ ending is changed to ‘ic’ acid. For example, when hydrogen chloride (HCl) dissolves in water, it forms HCl (aq), also known as hydrochloric acid. One exception is hydrogen cyanide (HCN), known as hydrocyanic acid. If an acid contains oxygen, it dissolves in water to form a hydrogen ion and a polyatomic anion containing oxygen. The most common type of oxygen-containing acid is denoted by the suffix ‘ic’ acid. It’s polyatomic anion’s name ends in ‘ate’. If the acid contains a polyatomic ion with an ‘ite’ ending, the name is changed to ‘ous’ acid.
Characteristics of Acids
The properties of acids can be listed as follows: –
- Acids have a sour taste
- Acid changes the colour of litmus paper from blue to red.
- Acids possess the ability to conduct electricity due to the presence of ions in them.
- Some acids also possess a corrosive nature and cause rusting of metals.
- Acids produce hydrogen gas in reaction with an active metal.
- The pH of acidic solutions is less than 7, with lower pH values corresponding to increasing acidity. Acetic acid, sulfuric acid, and tartaric acid are all examples of acids.
Definitions of Acids
Acids can be defined in three different ways. When someone says ‘an acid’, they usually refer to an Arrhenius or Bronsted-Lowry acid.
Lewis’s acids are commonly referred to as ‘Lewis acids’. The disparity in definitions is that these various acids do not contain the same set of molecules.
- Arrhenius Acid – An acid, according to this definition, is a substance that, when added to water, raises the concentration of hydronium ions (H3O+). As an alternative, one could increase the concentration of hydrogen ions (H+).
- Bronsted-Lowry Acid – An acid, according to this definition, is a material that can act as a proton donor. This is a broader definition because solvents other than water are not excluded. A Bronsted-Lowry acid is any compound that can be deprotonated, including acids, amines, and alcohol. This is the most common definition of an acid.
- Lewis Acid – A Lewis acid is a compound capable of accepting an electron pair and forming a covalent bond. Some non-hydrogenated compounds, such as organoboranes and boron trifluoride, are classified as acids.
Strength of Acids
- An acid’s strength is depicted by the ease it donates a proton, in different instances of expo solution. Stronger acids have a quick ability to be ionised and dissociate in a solution compared to weaker types.
- A stronger acid readily ionises or dissociates in a solution than a weaker acid.
- A strong acid, such as hydrochloric acid, dissociates completely into its ions in water. Because a weak acid only partially dissociates into its ions, the solution contains both water and ions and the acid.
Factors affecting the strength of the acids.
The factors affecting the strength of acid are as stated below: –
- The strength of the HA molecule
- The polarity of the molecule
These two elements are linked because the higher the molecule’s polarity, the more electron density is drawn away from the proton. The stronger the H-A bond and the more efficiently the proton dissociates in solution, the higher the partial positive charge on the proton.
Conclusion
The terms acid and base have been defined in various ways, depending on how the properties of acidity and basicity are viewed. Arrhenius initially described acids as substances that produce hydrogen ions when ionised, and bases as compounds that ionise to produce hydroxide ions. As per the explanation given by Lowry-Bronsted, an acid donates a proton and the base accepts the protons.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out