There are acidic and basic solutions all over the world. Except for water, virtually every liquid we come into contact with on a daily basis contains acidic and basic characteristics. Vinegar is a dilute solution of acetic acid, which belongs to the class of acids. An arrhenius acid is an acidic substance that produces hydrogen ions in solution.
Electrophilic substitution takes place when a pair of Π-bonded electrons attack an electrophile – usually carbocation species. In this way, the double bond is re-established in its original position through proton abstraction or by isomerization. There is electrophilic substitution involved in several benzene-containing reactions. There are a number of electrophilic aromatic substitutions among which nitration, sulfonation, halogenation, and Friedel-Crafts reactions are important.
Acidic nature
Several well-known acids (e.g., sulfuric, hydrochloric, nitric, and acetic acids) produce hydrogen ions in solution. The concentration of hydrogen ions produced determines whether these acids are strong or weak. A salt is formed when an acid reacts with a base; the product is the result of reaction between hydrogen ion and the hydroxide ion.
Types Of Acids
Acids can be either organic or inorganic. Mineral acids are inorganic acids. The strength of organic acids is generally less than that of inorganic acids. Inorganic acids do not contain carbon, which is the main difference between the two.
Inorganic acids or mineral acids can either be in a gaseous or solid form. Inorganic anhydrides are metalloid oxides that can combine with water to form inorganic acids. The following acids are examples: Sulphuric acid (H2SO4), Phosphoric acid (H3PO4), Nitric acid (HNO3)
The organic acids are toxic and corrosive. Acid corrodes tissue that comes into contact with it. Acids are used in all types of chemical productions. Examples include acetic acid, citric acid and formic acid.
What are Electrophilic Substitution Reactions?
A chemical reaction in which an electrophile replaces a functional group on a compound is known as an electrophilic substitution reaction. Hydrogen is usually the functional group that is replaced. Electrophilic substitution reactions are of two types, electrophilic aromatic substitution and electrophilic aliphatic substitution.
Mechanism for Electrophilic Substitution Reactions
There are three steps in the electrophilic substitution mechanism, which we will examine further.
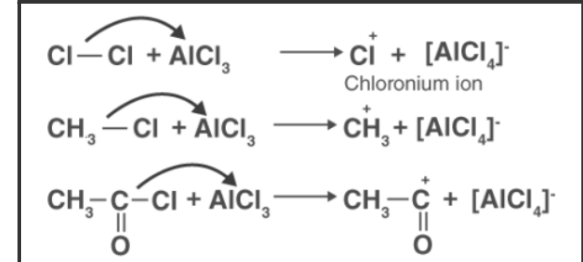
- Electrophile Generation:
Chlorination and acylation of an aromatic ring create electrophiles using anhydrous chloride. Electrophiles are produced by combining anhydrous aluminum chloride and attacking reagent, which are respectively Cl+, R+, and RC+O.
- Carbocation Formation:
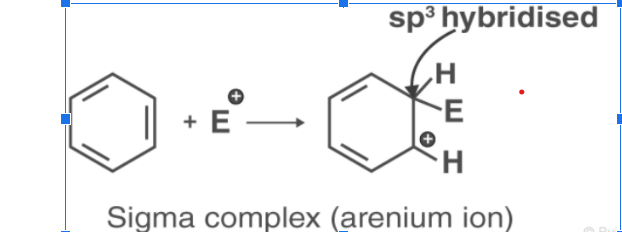
The amine is then attacked by the electrophile, forming the arenium ion or sigma complex. A carbon atom will be hybridized with sp3 hybridisation in the sigma complex.
Arenium ions or sigma complexes find stability in the resonance structure. Sigma complexes, however, lose their aromatic properties because electron delocalization stops at the sp3-hybridized carbon.
- Proton Removal:
This step restores the aromatic character of the carbon when the sigma complex or the arenium ion liberates an sp3-hybridized proton when AlCl4 attacks the carbon. The electrophile in the benzene ring replaces hydrogen in the third step.
Types of Electrophilic Substitution Reactions
Compounds undergo two primary types of substitution reactions electrophilic aromatic and aliphatic substitution reactions. See the example below.
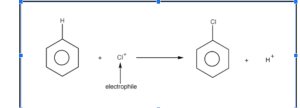
As an electrophile, the chlorine cation replaces a hydrogen atom in the benzene ring. An electrophilic substitution reactions leads to the formation of a proton and a chlorobenzene molecule.
Electrophilic Aromatic Substitution Reaction
A carbon atom on the aromatic ring is replaced with an electrophile in an electrophilic aromatic substitution reaction. An example of such a reaction would be aromatic nitration, aromatic sulphonation, or Friedel-Crafts reaction.
When aromatic compounds are substituted with electrophilic compounds, their aromatic properties are preserved. The aryl halides can therefore be obtained from aromatic rings and iodine, bromine, and chlorine through these reactions.
Electrophilic Aliphatic Substitution Reaction
When electrophilic aliphatic substitution reactions occur, the functional group (generally hydrogen) in the aliphatic compound is replaced by a functional group derived from the electrophile. There are five types of reactions.
- Halogenation of ketones
- Nitrosation
- Keto-Enol tautomerism
- Adding a carbene to a carbon-hydrogen bond
- Diazonium coupling (aliphatic)
If the electrophilic attack occurs from the rear (at an angle of 180 degrees to the leaving group), the electrophilic substitution reactions results in an inversion of the configuration.
What is Electrophilic Substitution Reaction of Benzene?
When an electrophile replaces the hydrogen atom of benzene, this is called an electrophilic substitution. Since this reaction does not affect the aromatic nature of benzene, it is highly spontaneous.
The Mechanism for Electrophilic Substitution reaction of Benzene
There are generally three steps in an electrophilic substitution reactions:
Electrophile generation:
Lewis acid contributes to the formation of electrophile. An electron pair is accepted by the Lewis acid from an attacking reagent.
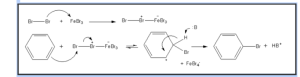
Arenium ion formation:
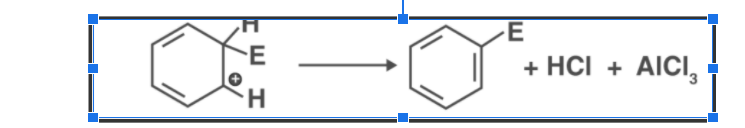
Electrophile attacks on the benzene ring result in the formation of a positively charged cyclohexadienyl ion. This ion carries one sp3 hybridized carbon atom. By resonance, three carbon atoms distribute the positive charge over them, making them partially stable.
Since an sp3 hybridized carbon atom stops the delocalization of electrons, the arenium ion is not aromatic.
- Discharge of the positively charged carbocation intermediate:
In the last step of the reaction, the arenium ion loses its proton and turns into a Lewis base, restoring its aromaticity.
Examples of Electrophilic Substitution Reactions
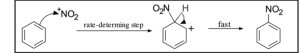
The nitration and halogenation of benzene are examples of electrophilic aromatic substitution. Electrophiles include Nitronium ions (NO2+) and Sulphur trioxide (SO3), which react with benzene separately to form nitrobenzene and benzene sulfonic acid, respectively.
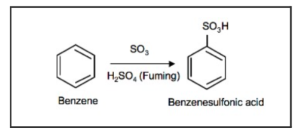
1.Benzene Sulfonation
Sulfuric acid fuming with benzene (H2SO4 + SO3) creates benzene sulfonic acid through the sulfonation of benzene. Reversible reactions occur in nature.
2.Benzene Nitration
In the result of protonisation of Nitric acid by Sulfuric acid, Nitronium ion is formed. This reaction takes place when there is loss of H2O.
3.Benzene Halogenation
Benzene reacts with halogens to form aryl halides in the presence of Lewis acids, such as FeCl3, FeBr3. We call this method “benzene halogenation.”.
4.Sulfuric Acid Activation of Nitric Acid
In this process, Nitronium ion is formed by activating HNO3 with sulfuric acid, which is a stronger electrophile.
Conclusion
In summary, Acidic nature of the compound is determined by the presence of H+ ions in the solution. More the concentration of H+ ions, the more acidic the compound is. On the other hand if we talk about Electrophilic substitution reactions, it is a chemical reaction in which an electrophile replaces a functional group on a compound is known as an electrophilic substitution reaction. Hydrogen is usually the functional group that is replaced. Electrophilic substitution are of two types, electrophilic aromatic substitution and electrophilic aliphatic substitution. Various examples of Electrophilic Substitution Reactions are Benzene Nitration, Benzene Halogenation, Benzene Sulfonation and Sulfuric Acid Activation of Nitric Acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out