Introduction:
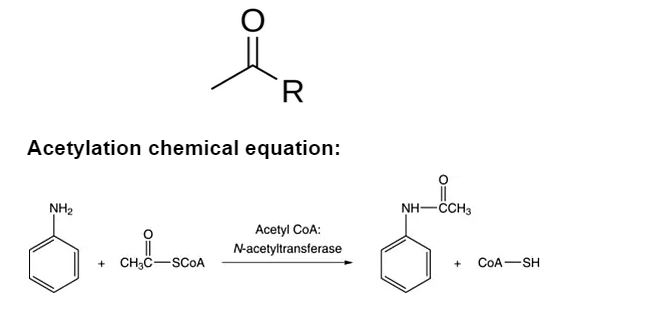
The addition of an acetyl group to a molecule is known as acetylation. A carbonyl group, or carbon doubly linked to oxygen, with a methyl group (CH3) at the end is what makes up an acetyl group. The acetyl group linked to your chemical is commonly denoted by the letter ‘R.’ For example, if we wanted to acetylate glucose, we could add an acetyl group to all of the nucleophilic alcohol groups (-OH groups). The acetyl group can also be abbreviated with the ‘Ac’ sign.
Salicylic acid reaction of Acetylation:
Acetic anhydride is used to acetylate salicylic acid, yielding acetylsalicylic (aspirin) and acetic acid as end products. Aspirin is also utilised in the manufacture of THC acetate ester and diacetylmorphine as an acetylating agent. The reaction is depicted in the diagram below.
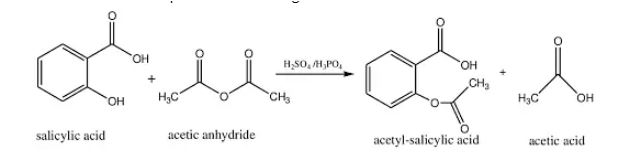
Salicylic acid is enabled in the presence of sulphuric acid because sulphuric acid aids in the separation of the acetate ion of the agent acetic anhydride. The process of dehydrogenation (removal of hydrogen atom) occurs when the acetate ion makes a stable connection with the nucleophilic atom of Oxygen (with its free electron) of salicylic acid, yielding acetic acid and aspirin.
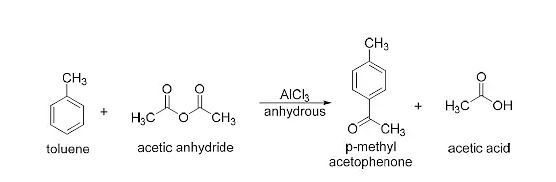
Acetylation with acetic acid:
The process of acetylation occurs when alcohols are treated with acetic acid at high temperatures. Toluene is employed as a solvent in this process, which produces an acetylated molecule.
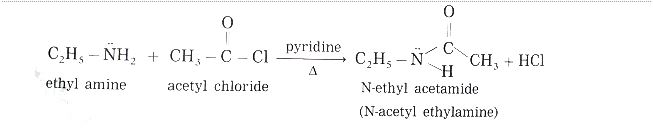
Acetylation with amines:
Several techniques of acetylation are carried out with the help of butyl acetate or ethyl acetate and acetic acids as catalysts and amines. At low room temperatures, such as 20°C, formamides are produced. The following chemical reaction is linked to the creation of broad-spectrum amines:
Dimethylacetamide is a recognised storage for acetyl and dimethylamine gas production. When easy acetylation with many amines occurs, it is treated at a temperature of 120 °C to 125 °C and used as an alternative in the rest of the acetylation calculations.
Acetylation of cellulose :
Because cellulose is a polyol, it is susceptible to acetylation with acetic anhydride. Acetylation impairs hydrogen bonding, which otherwise controls cellulose’s characteristics. As a result, the cellulose esters may be molded into fibers and films and are soluble in organic solvents.
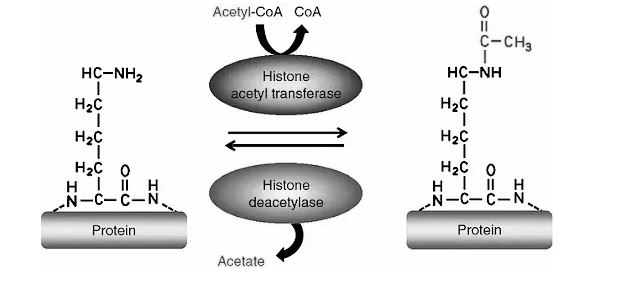
Deacetylation :
Deacetylation is the process of removing the acetyl group from a molecule in the opposite direction.Proteins that copy DNA for the purpose of mending genetic material that has been damaged must be created using acetylation to understand the accuracy and energy necessary for gene copying. The removal of acetyl groups is required for the use of lysine and various other gene-regulation functions. This is a brief process in which the lysine residues in the nucleosome’s N-terminal tail protrude from the histone core. It is acetylated and deacetylated because it is a crucial aspect of gene regulation. Acetylation of histones is thought to boost gene expression through activating transcription. As a result, the DNA becomes more accessible.
Acetylation reagents:
These three reagents are used to perform a variety of acetylations:
Anhydride of acetic acid: This reagent is widely used in laboratories, and it cogenerates acetic acid.
Acetyl chloride is a chemical compound. This reagent is also widely used in laboratories, although it produces hydrogen chloride, which can be harmful.
Ketene: Acetic anhydride was once made by reacting ketene with acetic acid as
What is O-Acetylation?
O-acetylation is the most common sialic acid alteration in nature, and it may be found in vertebrates, echinoderms, protozoa, fungi, and bacteria, much like sialic acids. The identification and characterization of O-acetylated sialic acids has progressed, and sialic acid-specific O-acetyltransferases (SOATs) and O-acetylesterases (SIAEs) that add and remove O-acetyl groups in mammalian cells, invertebrates, bacteria, and viruses have been found and characterized. These breakthroughs enable us to create genetically and biochemically engineered model cell lines and organisms with altered expression of O-acetylated sialic acids for dissection of their roles in glycoprotein stability, development, and immune recognition, as well as the discovery of novel functions.
Conclusion:
Acetylation is the process of organic esterification using acetic acid.
Deacetylation is the opposite reaction, in which the acetyl group is removed from a chemical molecule.Acetylation is used to make acetamides and acetate esters.
Acetic anhydride is the most popular reagent for acetylation in laboratories.
Ethanoylation is the name given to acetylation by the International Union of Pure and Applied Chemistry (IUPAC).Acetylation produces cellulose esters that are easily soluble in organic solvents and can be produced into films and fibres.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





