The full form of p-Nitroacetanilide is Para Nitroacetanilide. Nitroacetanilide is a combination of Nitro and Acetanilide. An organic substance, acetanilide, is processed through nitration to prepare p-Nitroacetanilide. It can be used to manufacture dyes, medicines, anti-oxidants, and gasoline, which have become essential in current times. However, the primary purpose of preparing the p-Nitroacetanilide is to obtain the p-Nitroaniline.
All aspects of p-Nitroacetanilide, including general information, preparation, and properties, are discussed in this curated p-Nitroacetanilide study material.
About p-Nitroacetanilide
p-Nitroacetanilide, or Para Nitroacetanilide, is an organic compound majorly used in pharmaceuticals and the production of dyes. Para Nitroacetanilide is the nitro derivative of acetanilide. Its chemical term is ‘C8H8N2O3’. The compound has a phenyl group that is connected to an amino group and is para (adjacent) to a nitro group. Hence, it is named Para Nitroacetanilide. Moreover, the compound is often known as 4-Nitroacetanilide, P-Acetamido Nitrobenzene, N-(4-nitrophenyl) acetamide and N-Acetyl-4-nitroaniline. Here’s a representation of P-nitroacentanilide.
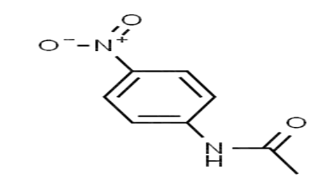
Before moving further, here is a summary of acetanilide. It is an organic substance produced by acetic anhydride with aniline. Acetanilide has no odour. Moreover, the chemical formula of the substance is C8H9NO. Acetanilide comprises some analgesic properties, owing to which, it is abundantly used in pharmaceuticals.
Preparation
As p-Nitroacetanilide is a compound and not found naturally, several unique methods and procedures are undertaken to develop the compound. Mainly, the p-Nitroacetanilide is prepared via nitration of acetanilide. In nitration, the acetanilide is processed and reacted via concentrated solutions of nitric and sulphuric acids.
Moreover, the glacial acetic acid is also used during preparation, as it possesses the properties of a polar solvent, which regulates the acetanilide dissolving. Additionally, ions of acetic acid are poor nucleophiles, which ensure that there will be no substitution reaction during dissolving.
In the overall process, an electrolytic substitution reaction would take place. Lastly, the acetanilide is purified via the process of crystallisation.
Reaction
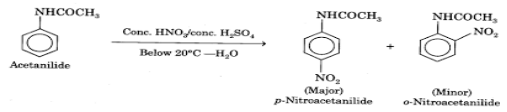
Theory
In the above Reaction, acetanilide (NHCOCH3)is nitrated with the concentrated nitric and sulphuric acid solutions. They result in two products of nitrified P-nitroacetanilide and o-Nitroacetanilide. After this, a purification process is processed to obtain pure P-Nitroacetanilide.
Purification of p-Nitroacetanilide (recrystallisation)
The first product, obtained from the reaction, contains mainly Para Nitroacetanilide or p-Nitroacetanilide. And the second product has a minor portion of Ortho Nitroacetanilide or o-Nitroacetanilide. However, o-Nitroacetanilide is a readily soluble substance. However, during crystallisation, it gets isolated in a flask while the P-Nitroacetanilide takes the shape of crystals and are separated with filter paper.
During the process, ethyl alcohol and ice isolate the o-Nitroacetanilide content. As a result, purified p-Nitroacetanilide is obtained, and the melting point is also determined.
Properties
- Para Nitroacetanilide appears as a solid or granular powder. Generally, it is slightly yellowish. Depending on the preparation, the colour can also be brown-green or green-yellow.
- The melting and boiling points of the compound are 215 °C and 409 °C (approx.) respectively.
- p-Nitroacetanilide has two isomers, namely, 2-nitroacetanilide and 3-nitroacetanilide.
- The molar mass of the compound is roughly 180.16 grams per mole.
- p-Nitroacetanilide is a good solvent. However, it is insoluble in cold water.
- p-Nitroacetanilide carries several analgesic properties which are used in pharmaceuticals. However, excessive consumption or exposure can lead to respiratory and skin problems. Also, it can lead to severe eye irritation.
Uses of p-Nitroacetanilide
As discussed earlier, p-Nitroacetanilide has analgesic properties which is helpful to the pharmaceutical industry to prepare several medications, like paracetamol. Moreover, it is used to develop poultry medicines, anti-oxidants, gasoline, pesticides, and rubber chemicals. Apart from this, a p-Nitroacetanilide hydrolysis reaction is carried out, and it is synthesised to obtain the P-nitroaniline.
It works as the reactant in a particular chemical reaction. The Reaction product is used further as a reactant in other Reactions to develop dyes. Lastly, p-Nitroacetanilide is also widely used in gum and corrosion inhibitors.
Conclusion
As we came so far, we learned all the aspects of p-Nitroacetanilide. From all the above, we learned that p-Nitroacetanilide is known as Para Nitroacetanilide, prepared after the nitration of acetanilide. And p-Nitroacetanilide is also an organic substance commonly used in pharmaceuticals for numerous purposes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



