Aluminum
Aluminum oxide is a crystalline powder that is white, odorless, and non-toxic. Insoluble in water. The manner of production affects the properties (both physical and chemical); different methods result in distinct crystalline modifications. Chemically, the variant generated at extremely high temperatures is inert.
Aluminum oxide (Al2O3), is a chemical compound made up of aluminum and oxygen with the formula Al2O3. It’s the most common of numerous aluminum oxides, and it’s known as aluminum(III) oxide.
Alumina is the most frequent name for it, however it can also be referred to as aloxide, aloxite, or alundum, depending on the form or application.
It occurs naturally as the mineral corundum in its crystalline polymorphic phase -Al2O3, which is used to make the precious gemstones ruby and sapphire. Al2O3 is used to make aluminum metal, as an abrasive because of its hardness, and as a refractory material because of its high melting point.
Structure
Corundum is the most frequent form of crystalline aluminum oxide, and it is also the most thermodynamically stable. With the aluminum ions occupying two-thirds of the octahedral interstices, the oxygen ions form a roughly hexagonal close-packed structure. The center of each Al3+ The molecule is octahedral.
Aluminum Chemical Formula
Al2O3 is the chemical formula for aluminum oxide. How did this formula come to be? To begin, we must remember that aluminum is a metal and oxygen is a non-metal, resulting in an ionic combination.
The aluminum ions and oxygen ions both have a charge. Aluminum has a positive charge of +3, whereas oxygen has a negative charge of -2. The formula for aluminum oxide is obtained by using the criss-cross method. In the chemical reaction, aluminum usually loses three electrons while oxygen acquires two, which is done primarily to achieve stability.
Solved Examples:
Q1. What is the oxide structural formula of aluminum?
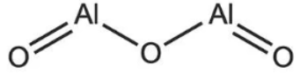
Q2. What are the properties of aluminum?
Although Al2O3 is an electrical insulator, it possesses a high heat conductivity for a ceramic material . Water does not dissolve aluminum oxide. Its hardness makes it appropriate for use as an abrasive and as a component in cutting tools in its most common crystalline form, corundum or -aluminum oxide.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




