To know in detail about the types of chemical bonds, you need to know what a chemical bond is. A chemical bond is an attraction between two or more ions, molecules, or atoms to form basic bonds or chemical compounds. There can be electrostatic force or electron sharing to make chemical bonds.
Not only this but there can be a bond between different states of matter and have different phases.
Based on these things, you can get two different strengths of bonds. They are primary or strong bonds and secondary or weak bonds.
Different types of chemical bonds
Based on different processes, there are different types of chemical bonds. Some share electrons, and some, donate the same. Chemical bonds are necessary to make elements more stable. There are various types from the very base of what is a chemical bond.
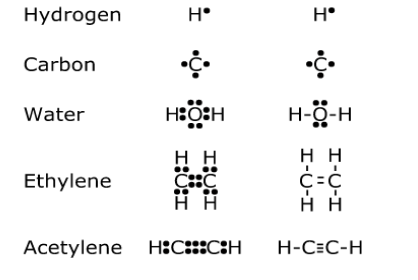
Octet Rules
The octet rule is nothing but to keep the last orbit of electrons with 8 electrons. When an element has 8 electrons in the last orbit, the element becomes low energetic and more stable. The same happens for hydrogen with two electrons, which is known as the duet rule. For this rule itself, chemical bonding happens.
Ionic Bond
The ionic bond is one of the most famous chemical bonds. This happens plenty of times and in an intelligent way. Many of our daily belongings have a popular ionic bond. The most common of our daily items, common salt sodium chloride (NaCl), is a very known example of an ionic bond.
According to bond definition chemistry, there must be one metallic and one non-metallic element in this bond. The metallic element will have fewer electrons in the valence orbit. Whereas the non-metallic will have more electrons. In the case of salt, the sodium (Na), the metal will donate an electron to chlorine (Cl), and the compound will form. This chemical compound is an ionic bond chemical compound.
Covalent Bond
In this case, you need to understand bond chemistry because here, both the elements share an equal number of electrons and make a bond. Water is an excellent example of a covalent bond. Here oxygen (O2) and hydrogen (H2) share an equal number of electrons.
One molecule of oxygen shares 2 electrons with two different molecules of hydrogen. This Chemical bond is made to get lower energy for both the elements.
Metallic Bond
This bond is only found when both the elements are metal. This process includes attractive electrostatic force between valence electrons. From the basics of what is a chemical bond, this process requires an electron cloud to create a chemical bond.
For this, detached and delocalised electrons are required. They will move freely, but ions and electrons interact to create a binding force to hold the metallic crystal.
The above three types of chemical bonds are responsible for strong bonds. This type of bond is also known as a primary bond.
Dipole-dipole interaction
Also known as dipole-dipole forces. This is a force between two different polar molecules’ positive and negative ends. Ideally, polar molecules have partial positive and negative ends. One positive end attracts the negative end of another polar molecule. Two nonmetal elements can have this kind of metallic bond.
Hydrogen bonding
Though this is a type of dipole interaction, there is a difference. In this chemical bonding, one polar molecule must be hydrogen. And that makes chemical bonding with oxygen, carbon, or nitrogen.
London dispersion force
Knowing what a chemical bond is will be very easy to understand. Sometimes two different atoms or molecules get attracted by symmetrical electron spreads. In this case, a chemical bond happens; although that’s a temporary dipole, it’s a different type of chemical bonding.
However, the last three types of chemical bonds cause weak chemical bonds. These types of bonds are also known as secondary bonds.
Conclusion
Despite having different types of chemical bonds, all the bonds are there to lower the element’s energy and make that more stable. With different types, you can get different strengths of chemical bonds.
The primary bonds are not that easy to break and less active towards any other reaction. However, the weak or the secondary ones are easy to break and more active towards further reactions.
From the beginning of what is a chemical bond to the different types of the chemical bond, you will know the various ways that a chemical bond works.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




