The periodic table is a grid structure containing all the elements known to humanity in order of increasing atomic number. Russian inventor Dmitri Mendeleev first devised this theory in 1871, and his classification is known as the first periodic law. The approach has gone through several changes for years as more complex elements were discovered, and a few have been found as recently as 2017. The modern periodic law is attributed to British physicist Henry Moseley, who was able to classify all elements into seven categories or “periods” of elements and the basis for this was the proton count in an element and not simply their atomic number.
The Periodic Table
As discussed above, in chemistry, the periodic table is considered the basis for classifying all the elements known to humanity with the ability to add more elements as and when they are discovered. There are seven periods in the table, which are laid out in order of their atomic number as well as a bias to the most the period in which it lies. There are two other subsections to the periodic table classification, containing lanthanoids and actinoids. There are both schools of 15 elements, each of which has varying atomic numbers which cannot fit into the periodic table and fall in the category of rare materials found on Earth.
There are, namely, five different ways in which the elements in the periodic table can be classified. It is important to understand why the classification of families and groups within the periodic table exists. This is due to the fact that elements are categorized by their atomic number and their resemblance to a similar element. All of these classifications help us understand where the element lies in the periodic table and what compositions it is capable of so that chemists and scientists can easily identify an element and its underlying properties simply by understanding the classification of the periodic table.
Let’s have a look at the five main element families that are available.
Broad Generalization of Elements
Alkali metals
Alkaline earth metals
Transition metals
Halogens
Noble gases
The elements can also be further categorized by the measure of composition
Alkali Metals
These are metals that give off a shine and are luminous in nature. They contain one electron giving them a low density but higher electrical conduction power. This also means that they possess a higher atomic mass. The element Hydrogen is not primarily from the Alkali metal family; however, under the right atmospheric conditions, Hydrogen can be cooled into a shiny flowy liquid that is considered alkali metal. Examples are Lithium and Sodium.
Alkaline Earth Metals
These are again metals with high density. They are far denser than the alkali metals and oxidize very quickly. They are far more potent as well and possess very high melting points. It has a high reaction and energy release when in contact with water when heated up. Examples are magnesium and Barium.
Transition Metals
These are a category of highly transitive metals. They can change their state of oxidation very fast and are high conductors of thermal and electrical energy. They are equally as dense and strong as alkaline Earth metals; however, their melting point is even higher, and they contain a measure of two electrons per nuclei. Examples are iron, copper and Zinc.
Boron Group or Earth Metals
This family contains both metals and nonmetals. They have powerful electrons in their nuclei. Example – Boron
Carbon Group or Petrels
They carry up to 4 charges in their nuclei. Contains both metals and nonmetals. Example – Carbon
Nitrogen Group or Pnictogens
These are primarily gases and have high powered energy dispersal solutions when cooled down to liquid form.
Oxygen Group or Chalcogens
The oxygen family of elements is the most predominant element vital for human consumption.
Halogens
This is a group of nonmetals that are nonreactive. They have a difference in their atomic number as they change from solid to liquid to gas. Example – Iodine
Noble Gases
They have compounding properties but are generally considered nonreactive nonmetals in their raw state. Famous examples are helium, xenon and radon.
This table below describes the different families or categories of the periodic table, color-coded for better understanding. The bottom two families are standalone and do not fall into the regular table categories.
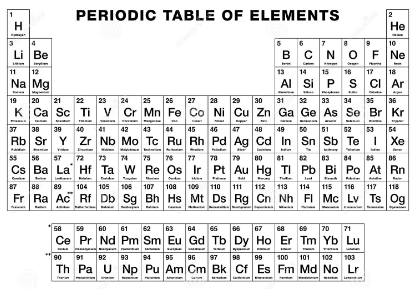
Conclusion
Periodic law is one of the essential concepts in the study of chemistry. Every chemist or chemistry enthusiast uses the periodic table for various purposes, whether consciously or subconsciously. This table lists the atomic number, proton valency, properties, reactive capabilities and fusion possibilities for every element known to humankind. From our local chemists to nuclear scientists, all use this system of periodic law to find an informative and correct way to approach any matter that requires the prowess of chemical history. We owe a great deal to Dmitri Mendeleev and Henry Moseley to create this law and can also look forward to remarkable inventions and discoveries in the field of chemistry.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




