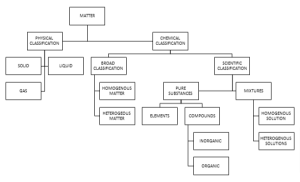
What is the matter? Matter is the basic entity that makes us every object present in the universe. The traditional classification of the matter is its division into the five fundamental elements colloquially known as ‘Pancha tattvas’. They include air, earth, fire, sky and water whereas the modern classification as per the scientist categorises matter based on its physical and chemical characteristics. Solid, liquid and gas are the three fundamental states of matter based on their physical characteristics. The classification of matter based on chemical characteristics is pure substance and mixtures.
CLASSIFICATION OF MATTER
Matter can be classified into two parameters:
- Chemical classification of matter
- The general classification of the matter is into Homogenous material and Heterogeneous material.
- The more scientific way of chemical classification of the matter is into Pure substances and Mixtures.
- Pure substances can further be classified into elements and compounds (both are homogeneous in nature).
We can further classify compounds into two parts i.e. inorganic and organic compounds.
- Mixtures can be classified as homogenous and heterogenous types of mixtures.
- Physical classification of matter
- Solids
- Liquids
- Gas
MATTER – ITS CHEMICAL NATURE
- Homogeneous matter: When the matter possesses both identical characteristics and uniform composition, the matter is said to be homogenous.
Example: water, motor oil (with a large number of hydrocarbons), iron etc.
- Heterogeneous matter: when the matter is present in several phases, we say it to be a heterogeneous material.
Example: a mixture of ice and water, iron and sulphur etc.
- Here, phase refers to a distinct portion of matter with uniform composition and properties.
- Different phases share different boundaries
WHAT ARE PURE SUBSTANCES?
A more scientific approach to describing the chemical classification of the matter is
- Pure substances:
- Element: It is that form of a pure substance that comprises only one type of particle. The particles could be atoms or molecules. An element cannot be broken into fragments nor can be built by any means of a physical or chemical process. It has definite physical and chemical characteristics. Elements can be further classified into Metals, Metalloids and Non- metals.
A few examples of elements include carbon, gold, mercury, hydrogen, oxygen, nitrogen, iron, lead etc.
- Compound: It is a pure substance comprising more than one variety of elements or atoms. The combination of all the particles is in a fixed proportion of mass and unlike elements, they can be separated into their constituent elements by employing physical and chemical methods. Compounds are majorly classified as organic and inorganic compounds.
A few examples of compounds include sulphur dioxide, carbon dioxide, caustic soda, washing soda, baking powder, sulphuric acid, hydrochloric acid etc.
- Mixtures: the constituents of mixture unlike compounds, can be present in any ratio or proportion i.e. not fixed. They have a non-uniform composition. The properties exhibited by the mixtures are someway in the middle concerning their constituents. Homogeneous mixture and heterogeneous mixture are the two varieties of the mixture. A few examples of the mixture are smoke (a combination of carbon particles suspended in air), gun powder (a combination of carbon, nitre and sulphur), oil in water etc.
A BRIEF – TYPE OF MIXTURE
- COLLOIDAL SOLUTION: The particles have a uniform distribution throughout the colloidal solution. As compared to suspension, the particles of the colloidal solution have a relatively small size mimicking a homogeneous mixture. But actually, it is a heterogeneous mixture, for example, milk.
- SOLUTION: It is considered a homogenous mixture containing two or more substances in it. The particles in a solution show homogeneity in their composition. A lemonade drink would have the same taste.
Solutions could be solid or gaseous solutions. For instance, an alloy is an example of a solid solution whereas the air around us is an example of a gaseous solution.
- SUSPENSION: It falls under the category of a heterogeneous mixture. Scattering of light is possible through a suspension.
MATTER – ITS PHYSICAL NATURE
The existence of the three states of matter is due to equilibrium achieved by thermal energy and intermolecular forces prevailing in solids, liquids and gaseous phases.
- SOLID: A substance is said to be in a solid form when disruptive thermal energy is predominated by high intermolecular energy.
- LIQUID: It is an intermediate phase existing between solid and gaseous phases.
- GAS: It is the intermediate phase that exists between the liquid and plasma state of matter.
PHASE TRANSITIONS
The states of matter undergo transition among themselves by the following process:
- Melting: transition from solid to liquid state
- Sublimation: transition from solid to gaseous form.
- Freezing: transition from the liquid state to a solid-state
- Vaporisation: transition from the liquid form to the gaseous form.
- Condensation: transition from gas to liquid phase
CONCLUSION
All the objects existing in nature have their basic composition as matter. The modern classification system classifies matter into two categories based on physical traits and chemical traits exhibited by the particles. Physical classification of the matter is into solids, liquid and gases. Chemical classification of matter includes firstly homogeneous and heterogeneous matter and secondly, it comprises majorly pure substances and mixtures. A pure substance contains only a single kind of particle. Simply, a substance exists as a pure variant of matter. A pure substance can be further divided into elements, mixtures and compounds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




