Answer: The colour of copper sulphate when an iron nail is kept in it changes from blue to pale green because iron is more reactive than copper thus displacing copper from the solution resulting in colour change.
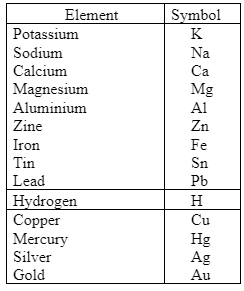
The metal activity series is an empirical method for predicting products in displacement reactions and metal reactivity with water and acids in replacement processes and ore extraction. The activity series is a table that lists metals in decreasing order of relative reactivity. The metals at the top are more reactive than those at the bottom having a less reactive nature. From most reactive to least reactive, the reactivity sequence is as follows:
When an iron nail is dipped in copper sulphate solution originally in blue colour, the surface of the iron develops a brown copper coating, and the colour of the copper sulphate solution changes from blue to pale green as the iron is dissolved in the solution as Fe (II), producing ferrous sulphate FeSO4.
The reaction demonstrating the given chemical activity is:
CuSO4(aq)+Fe(s)→FeSO4(aq)+Cu(s)
Here, a redox reaction is taking place,
(1) Reduction process Cu2++2e → Cu0 and Cu2+is an oxidising agent.
(2) Oxidation process Fe0-2e → Fe2+ and Fe0is a reducing agent.
This shows that the reactivity series has several key applications aside from offering insight into the characteristics and reactivities of metals as it can be used to forecast the outcome of reactions between metals and water, metals and acids, single and double displacement processes between metals.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






