Answer: Carbonate has a valence of two because of its property of combining with 2 hydrogen atoms.
The concept of valence and valency is related to the electrons in an atom’s outermost orbital. Although there is a major distinction between valence and valency, that valence refers to an atom’s ability to join with another atom, whereas valence refers to the maximum amount of electrons that an atom can lose or gain in order to maintain stability.
One of an atom’s valences is equivalent to the valency of that atom because an atom’s combination strength is determined by the greatest amount of electrons it can lose, gain, or share. As a result, despite the differences in definitions, the value of valence and valency can be the same which is also the case in carbonate ion CO3-2.
The mineral carbonate is a member of the mineral family that contains the carbonate ion CO3-2, which serves as the mineral’s compositional and structural unit.
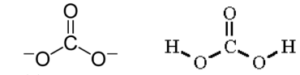
With one of the three O atoms, carbon with valency 4 makes a double bond, while with the other two O– ions, it forms a single bond resulting in a carbonate structure. The three atoms of oxygen interact dynamically with carbon, forming Lewis resonance structures that give the ion more stability.
Thus, we can conclude that
- Carbonate has a valence of two because it may interact with two H atoms
- Due to the negative charge of each oxygen atom, it can interact with an H+ ion to generate carbonic acid
- Carbonate is an anion with a -2 charge and -2 valency as it gains two electrons to become a stable compound
Carbonates are used as raw materials in a variety of industrial processes, including drug development, glass production, pulp, and paper production, sodium chemicals (silicates), soap and detergent production, paper production, and water softener production, clay and concrete production, to name a few.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






