Q. What is the valency of sulphate (SO42-)?
Answer:- Valency refers to the number of electrons that are gained or lost by an atom in order to complete its outermost shell. By completing their outer shell, or octet (8 electrons in the outmost shell), the atoms become stable.
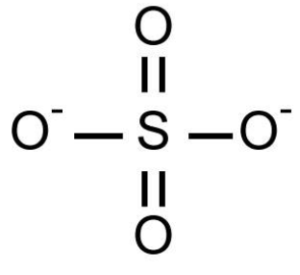
Sulphate has the formula SO42- which makes it a sulphur-oxygen compound. Potassium, calcium, sodium, barium and magnesium are some of the elements that form salts with sulphate. Water readily dissolves ionic sulphates. Sulphates are abundant in nature and can be easily manufactured in the industrial setting. The structure of chemical bonding of sulphate ion is:
Now, the valency of sulphate SO42- which is an anion is derived from sulphuric acid (H2SO4). The valency of hydrogen in H2SO4 acid is 1. Because hydrogen is more electropositive than SO42-, its oxidation number will be +1. Using this information, we can compute the oxidation number of SO42-.
Let’s call x the oxidation number of SO42-. It is equated with zero since the total of oxidation numbers is zero.
H2SO4 = 2×1 + 1×SO4
0 = 2+1 × x
x = -2
The oxidation number of -2 shows that sulphate gained two electrons to complete its octet indicating its valency as 2. Thus, valency of SO4 is 2.
Some applications of different compounds of sulphates are:
In therapeutic baths, magnesium sulphate is beneficial.
Metal salts can be made with the help of sulphate minerals.
Copper sulphate is also one of the most prevalent and fundamental algaecides.
Detergents, emulsifiers, and foaming agents can all benefit from these.
Gypsum is a naturally occurring kind of hydrated calcium sulphate that we use to make plasters.
These come in handy during the construction process.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out