Answer:
The ratio of the moles of a component present in the solution to the total number of moles of all the components in the solution is called the mole fraction of that component. While molality is termed as the measure of the number of moles of solute in the solution that corresponds to 1000g or 1kg of solvent
Mole fraction= number of moles of solute
number of moles of solvent
mole fraction= nB / nA + nB
Molality= moles of solute / Moles of solvent
Molality= nB/WA
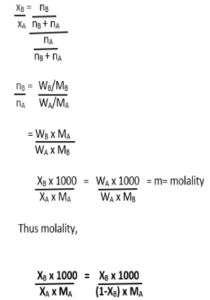
Relation between mole fraction and molality-
Let us consider a solution with
A= solvent and B= solute.
So,
Mole fraction of solvent = xA
Mole fraction of solute = xB
Number of moles of solvent = nA
Number of moles of solute = nB
Mass of solvent = WA
Mass of solute = WB
Molar mass of solvent = MA
The molar mass of solute = MB
Mole fraction of solute = xB = nB / nB + nA
Mole fraction of solvent = xA = nA /nB + nA
Now, let us divide equations (1) and (2)
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






