Answer: The molecular mass of a chemical compound is defined as the mass of a sample (in moles) of that compound divided by the quantity of chemical substance in that sample. The molar mass is a mass property of a substance, not a molecular property. The molecular mass is usually expressed in amu.
The relative atomic masses of each element present in a molecule can be added to determine the molecular mass of that compound. Also, the chemical formula can be used to determine the number of atoms in a molecule.
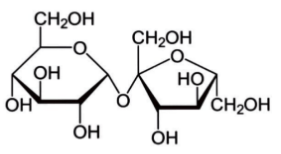
Cane sugar molecule is the composition of glucose and fructose which are two monosaccharides resulting in a disaccharide. Its structure is:
The steps to be followed to find cane sugar molecular mass are:
- Cane sugar’s molecular formula is C12H22O11 according to which we can find the number of atoms in a molecule.
- Next, using the mass of carbon, hydrogen, and oxygen given in the Periodic Table, calculate the atomic mass of each element.
- Then, multiply the subscript representing the number of atoms by the atomic mass of that element.
- Add the atomic masses of all the atoms in the molecule to get the molecular mass.
Thus, when the atomic masses of carbon, hydrogen and oxygen are rounded to four significant figures, the molecular mass will be:
C12H22O11 =12( Atomic mass of C)+22( Atomic mass of H)+11( Atomic mass of O)
C12H22O11=12(12.01)+22(1.008)+11(16.00)
C12H22O11=342.30 amu .
Sucrose or Cane sugar is extracted and refined for human use from either sugarcane or sugar beet. Sugar mills smash sugarcane to produce raw sugar, which is then shipped to other businesses to be refined into pure sucrose. They are frequently found in tropical areas near sugarcane farms.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out