Answer: Isomerism is a concept and term coined by Swedish scientist Jacob Berzelius in 1830 to describe the phenomena of two or more chemical compounds having the same molecular formula.
Stereoisomers are chemical compounds with an identical molecular formula that differ solely in the arrangement of their atoms in three-dimensional space. Constitutive isomers and stereoisomers are the two types of isomers, and the stereoisomer category contains multiple subclasses. Let’s look at the two primary forms of stereoisomers in more detail:
- Geometric Isomers: Geometric isomers, commonly known as cis/trans isomers, are stereoisomer types that result from a double bond or a ring structure.
- The cis and trans isomers of alkenes of the type R–CH=CH–R exist.
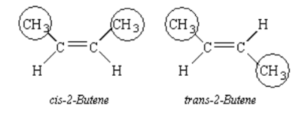
- The geometric isomers cis -2-butene and trans -2-butene is the most basic. The double bond in each molecule lies between carbons 2 and 3.
- The methyl groups connected to carbons 2 and 3 are on the same side of the stiff double bond in cis -2-butene.
- The methyl groups in trans -2-butene are on opposite ends of the double bond.
2. Optical Isomers: Optical isomers are molecules with mirror images of their structures that cannot be overlaid in any direction.
- Chiral substances are molecules that are not super-imposable mirror images of one other, and this quality is known as chirality.
- Symmetrical plane does not exist in optical isomers.
- Thus, optical isomers are known as chiral. Enantiomers are the d and l isomers of a compound.
- In octahedral compounds with 1, 2, or 3 symmetrical bidentate ligands, optical isomerism is widespread. [Co(Cl)2(en)²]+, for example.
As explained in geometric isomers, the most prevalent example of stereoisomer is cis -2-butene and trans -2-butene.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






