The Hybridisation of NiCO4 is:
A. dsp²
B. sp²
C. sp³d
D. sp³
Answer: (D)
Explanation: Nickel is present in NiCO4 in the zero oxidation state Ni = 3d84s². Because CO is a powerful ligand, it can pair the unpaired electrons. As a result, the 4s electrons are assigned to the 3d orbital. The 3d orbital is now totally filled, while the 4s and 4p orbitals remain open. Because these four orbitals form a degenerate set, hybridization is sp3 hybridised.
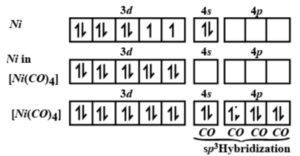
- A ligand is an ion or molecule that forms a coordination complex by donating a pair of electrons to the central metal atom or ion. The word ligand comes from the Latin word ligand, which means “to tie or bind.” Anions, cations, and neutral substances can all be used as ligands.
- A strong ligand, often known as a strong field ligand, is one that can cause more crystal field splitting. This indicates that when a strong field ligand binds, the differential between the higher and lower energy level orbitals increases. CN– (cyanide ligands), NO2– (nitro ligands), and CO– (carbon ligands) are some examples (carbonyl ligands).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






