Answer:- (A) True
Explanation:
Sodium Carbonate is manufactured largely by the Solvey process. The raw materials required for this process are salt brine (NaCl), ammonia (NH3), and limestone (CaCO3).
In this process, the sodium carbonate is obtained as follows:
Carbon dioxide and ammonia are passed into a cold saturated solution of sodium chloride. The reactions occur in the solution and thus, ammonium and hydrogen carbonate is formed.
The hydrogen carbonate formed is very slightly soluble and in the presence of sodium ions, it is completely precipitated. The addition of common salt to the solution containing NH4+ and HCO3– precipitates NaHCO3. Since it is the least soluble, it is filtered off.
The sodium bicarbonate obtained is heated to gain the final product sodium carbonate which is sodium carbonate Na2CO3.
Sodium carbonate Na2CO3 is a diazonium salt of carbonic acid which forms carbonic acid and sodium hydroxide when dissolved in water.
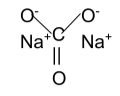
The structure of sodium carbonate is:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out