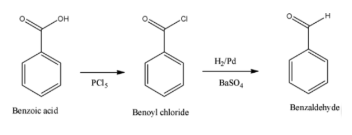
Answer: The steps for conversion from benzoic acid to benzaldehyde are:
- The very first step of the reaction is converting benzoic acid to benzoyl chloride with the help of phosphorus pentachloride.
- Then, a chlorination reaction occurs when this reagent changes an acidic group to a chloride group.
- Now, because the -Cl group is a better group to get removed, it may easily undergo a reduction process in the presence of Lindlar’s catalyst, which is composed of H2/Pd, BaSO4.
- Therefore, when the benzoyl chloride is reduced with Lindlar’s catalyst, the required product i.e., benzaldehyde is obtained. This step is known as Rosenmund’s reduction . The reaction in structural form is as shown below:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






