The nitrobenzene resonance structure is drawn as
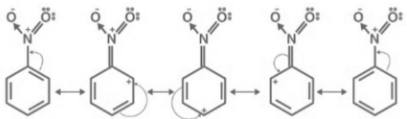
The first nitrogen is joined to benzene with a double bond, while one oxygen is attached with a single bond, according to the first structure.
- Now, because nitrogen has a low electron density, it tries to obtain electrons, according to the second structure. Therefore, the bonds are sifted out, and nitrogen receives an electron.
- Both oxygen atoms have a negative charge, as a result.
- Similarly, single bonds, double bonds, and +ve charges are used in every structure.
- 1st structure and 5th structures are identical in the resonance structure which indicates the final structure is made from resonating.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






