Draw a molecular orbital diagram of N2or O2 with magnetic behaviour and bond order.
A molecular orbital (or MO) is an orbital in the atomic structure of molecules. It is a molecule’s electron wave function that is used to calculate its chemical and physical properties.
Molecular orbital diagram of nitrogen molecule:
Given element – Nitrogen
Atomic number of nitrogen – 7
Electronic configuration – 1s²2s²2p³
Total number of electrons in nitrogen molecule – 14
Molecular orbital diagram of N2 is as follows;
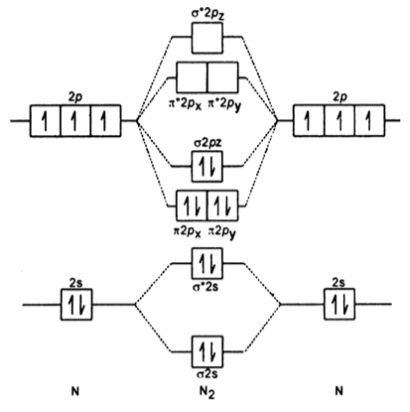
Electronic configuration: ó 1s² < *ó1s² <ó 2s² < *2s² , [ π2px² = π2py²] < ó 2pz ² < [*π2px =*π2py] < *ó2pz
Let’ s calculate the bond order of N2;
Bond order = Bonding electrons – Anti bonding electrons / 2
= 10 – 4 / 2 = 3
Therefore, the order of N2 is 3.
N2 does not have unpaired electrons, hence it is diamagnetic.
Molecular orbital diagram of oxygen molecule:
Given element – Oxygen
Atomic number of oxygen – 8
Electronic configuration – 1s²2s²2p4
Total number of electrons in nitrogen molecule – 16
Molecular orbital diagram of O2 is as follows;
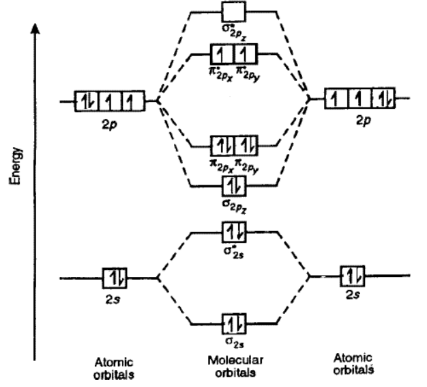
Electronic configuration of oxygen molecule;
ó1s² < *ó1s² < ó2s² < *ó2s² , [ π2px² = π2py²] < ó 2pz² < [*π2px¹ =*π2py¹] < ó*2pz
Let’ s calculate the bond order of O;
Bond order = Bonding electrons – Anti bonding electrons / 2
= 10 – 6 / 2 = 2
Thereofore, the order of O2 is 2.
O2 have unpaired electrons, Hence it is Paramagnetic.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






