Define Anti Markovnikov Rule And Markovnikov With Example.
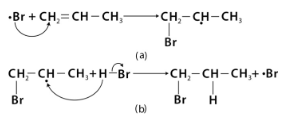
Anti Markovnikov Rule:
The Anti-Markovnikov rule defines regiochemistry in which the substituent is bound to a less substituted carbon rather than the more substituted carbon. Because substituted carbocation allows for more hyperconjugation, the carbocation becomes more stable.
In the presence of peroxide, HBr is added to unsymmetrical alkenes, forming 1-bromopropane instead of 2-bromopropane.
The alkene is attacked first by the Br radical. Because the carbon radical generated will be at the more substituted carbon, it attacks the less substituted carbon for increased stability.
The carbon radical then attacks another HBr molecule’s hydrogen, releasing another Br radical, and the reaction continues.
Markovnikov Rule:
According to this, when any group is added to an unsymmetrical alkene, the negative half of the reagent attaches to the carbon atom with the least amount of hydrogen, while the hydrogen attaches to the carbon atom with the most hydrogen.
When a protic acid (HBr) is introduced to an asymmetric alkene, the acidic hydrogen connects to the carbon atom with the most hydrogen substituents, while the halide group attaches to the carbon atom with the most alkyl substituents.
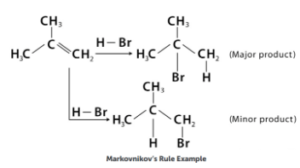
In an asymmetrical alkene addition reaction, Markovnikov’s Rule tells us where to add the nucleophile and hydrogen. When researching alkene addition reactions, it’s important to comprehend and recognise this pattern.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out