Mass spectroscopy is one of the techniques used to determine or quantitate the molecular weight and the functional elements present in the molecule. It is technically not a spectroscopic technique as it does not use the principle of light absorption as it is in the spectroscopic methods. Generally, mass spectrometry consists of a collection of mechanisms.
One of those mechanisms is the rearrangement of the bonds and electrons in the molecule. The McLafferty reaction showcases the rearrangement in Mass Spectrometry. The rearrangement mechanism in the Mass Spectrometry is briefed below.
Mass Spectrometry
The evaluation of the molecular weight and the functional group present in a molecule through mass spectrometry is based on the Mass/Charge(m/z) value of the dissociated molecule.
Process of Mass Spectrometry
The process of mass spectrometry includes the following sequential steps.
- The molecule in the sample is struck with an electron beam.
- The electron beam ionises the molecules in the model; that is, the molecules lose an electron or two, becoming ions.
- The resulting ions are characterised based on their Mass/Charge values, which the detector notes based on their deflection angle.
- The result is a Mass Spectrum detailing the molecule’s nature with unique peaks, representing the corresponding functional groups in the molecules.
Mass spectrum
- The mass spectrum is the result of mass spectroscopy analysis.
- It is a graph that contains the mass to charge ratio of the ionic molecules in the sample marked against their concentration.
- When the height of the peak is higher, it denotes that the concentration of the individual molecule is more.
- The mass to charge ratio is unique to every molecule containing functional groups.
- The value changes according to the nature, bondage, and position of the functional group in the molecule.
Rearrangement in Mass Spectrometry
The McLafferty rearrangement
- The ionic molecules resulting due to the electron beam are not energetically stable.
- Thus, they undergo the shifting of bonds and electrons within them.
- It is referred to as rearrangement, mainly termed as McLafferty rearrangement.
- As a result of the rearrangement of the bonds within the molecule, the molecule breaks into two products: enol and alkene.
- Enol is a compound containing an (-OH)alcohol and a double bond.
- Alkene is a molecule consisting of unsaturated double bonds.
- The resulting enol is electrically charged, whereas the resulting alkene is neutral without any charge.
- As it is neutral, the alkene is not calculated for the mass spectrometry detector, whereas the ionic enol is considered.
- As per the mass to charge ratio(m/z) of the enolic groups, the molecule present in the sample is characterised and identified.
Rearrangement mechanism
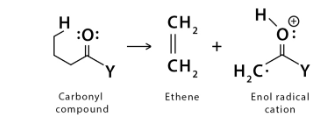
- Let’s consider the substrate molecule in the sample as the one given on the left side of the reaction. On ionisation, the right side product is yielded.
- The product starts undergoing McLafferty rearrangement as it is chemically unstable. The bond (the electrons) from the gamma hydrogen (hydrogen present on the third carbon atom) shifts to the unsaturated carbonyl group (-C=O).
- After receiving the electrons from the gamma hydrogen, the oxygen of the carbonyl group shifts its free electrons to its carbonyl carbon (the first carbon, the carbon present in the ketone functional group).
- Due to a double bond in the carbonyl group already, the electrons are shifted to the alpha carbon (the second carbon, the carbon attached to the functional group, here, the carbonyl ketone group).
- The molecule is still not stable; thus, the electrons are further shifted to the beta carbon (the third carbon, the carbon next to the alpha carbon) to attain better stability.
- Yet, the beta carbon shifts the electrons to the gamma carbon (the carbon next to the beta carbon, the 4th carbon).
- For further stability, the molecule is cleaved at two places: one cleavage at the gamma hydrogen and the other at the beta bondage.
- On cleavage, the products are, as already mentioned, an alkene and an enol.
The type of molecules that undergo rearrangement
The molecules that follow the mechanism of the McLafferty rearrangement should possess the following characteristics.
- The molecule should have equal to or more than four carbons to give gamma hydrogen to the reaction.
- The molecule must have at least one pi electron to contribute to the electron shift to achieve stability.
- It must have free electrons in the functional group to receive the gamma hydrogen electrons, shifting their free electrons to its adjacent carbon atom.
Conclusion
Mass spectrometry involves charging the molecules electrically in the given sample to identify them. As a result of ionisation, the molecules become energetically unstable, thus undergoing McLafferty Rearrangement in mass spectrometry. It causes a rearrangement mechanism of bonds and electrons in the molecule. Consequently, they form an alkene and enol as products. Of these two products, the ionically-charged enol is detected by the spectrometer. The resultant mass spectrum gives the molecules present in the sample by analysing the peaks formed according to the mass to charge ratio of the molecular ions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






