The Wolff-Kishner reduction is a generic process for removing a carbonyl group from alkanes. Ethylene glycol, a high-boiling-point solvent, is commonly utilised to achieve the high temperatures required for this reaction. Hydrazine is converted into nitrogen gas as the reaction proceeds. This process is employed to reduce aldehydes and ketones. Other reducible compounds are typically unaffected by the reaction conditions. This is an organic process that converts aldehydes and ketones into alkanes. Some carbonyl compounds can be quickly reduced to alkanes because they are viable in strongly basic circumstances.
All About the Wolff-Kishner Reduction
Over a century has passed since the Russian scientist Nikolai Kishner in 1911 and German scientist Ludwig Wolff in 1912 discovered the Wolff-Kishner reduction reaction.
The Wolff-Kishner conversion reduces a carbonyl group into hydrocarbons when heated in the presence of a hydrazine hydrate reagent and a base. Through >C=N, the carbonyl group (>C=O) is transformed to a >CH2 group in this procedure. The release of NH2 and nitrogen gas occurs. Carbonyl groups are converted into methyl groups in this process. Ethylene glycol, a high-boiling-point solvent, is needed to remove the nitrogen gas that forms as a side product of the process.
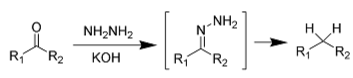
Other reducible groups, such as double bonds between carbon atoms and nitro groups, are generally unaffected by the settings of this reaction.
The carbonyl compound is heated to 180°C in a sealed tube with hydrazine hydrates and sodium ethoxide as the initial step in the reduction.
Mechanism
The Wolff-Kishner reduction pathway begins with creating a hydrazone anion, which then releases an atom of nitrogen to produce a carbanion. This carbanion subsequently reacts with the system’s water to produce a hydrocarbon. This approach typically uses diethylene glycol as a solvent.
Step 1
Szmant and colleagues explored the Wolff–Kishner reduction mechanism. According to Szmant’s research, creating a hydrazone anion, 1, is the initial step in this reaction, caused by the dissociation of the terminal nitrogen by MOH. Whenever semicarbazones are used in the molecule synthesis of hydrazones, they must first be transformed into the matching hydrazone before being deprotonated. Several mechanistic studies suggest that the delocalised hydrazone anion’s carbon terminal forms a new carbon-hydrogen bond.
Step 2
In the case of nitrogen atoms near the end of a chain, deprotonation occurs, and a double bond is formed with one of the nitrogen atoms next to it. As a result, the hydrogen’s fundamental environment attaches to the reaction’s proton to generate water.
Step 3
Water molecules protonate carbon because oxygen becomes less electron withdrawing than carbon, hence protonated carbon.
Step 4
It produces a triple bond between the terminal nitrogen and its neighbouring nitrogen. The carbanion releases the three triple-bonded nitrogen atoms that produce nitrogen gas. Similar to step 2, the proton and its fundamental surroundings are ejected from the system.
Step 5
In the next step, a protonation reaction occurs, resulting in a hydrocarbon product. Consequently, aldehydes and ketones are transformed into alkanes.
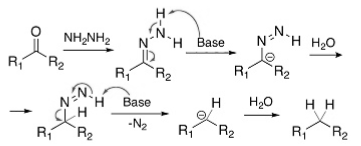
A hydrogen connection between terminal carbon and the hydrazone anion controls this process. Substituents with weak electron-withdrawing properties facilitate the establishment of carbon-hydrogen chemical bonds. Nitrogen substitutes with significant electron-withdrawing capabilities reduce the negative charge of nitrogen, making it far more difficult to break the N-H bond than other nitrogen substitutes.
Wolff-Kishner reductions can be transformed into various techniques, each with advantages and drawbacks.
Conversion into Alkanes
There is a process called deoxygenation that removes a carbonyl group. For example, the double bond of carbon and oxygen is changed to a methylene group in this process. Wolff-Kishner reduction is one of these reactions.
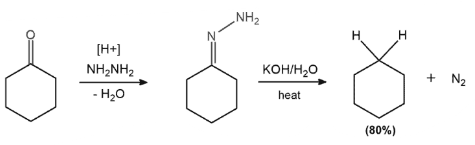
Simple hydrazones are formed by condensing hydrazine with aldehydes and ketones, which decompose at high temperatures, releasing nitrogen gas. Hydrocarbons are formed as a result of this process.
Hydrazone is first protonated by the base (hydroxide), producing the equivalent anion, stabilising through resonance. Next, water protonated this anion, resulting in the formation of a neutral azo-intermediate. Finally, the base takes another proton from the intermediate, resulting in the formation of an anion with a leaving nitrogen group that is highly effective. This anion decomposes swiftly and irreversibly due to its ability to extract nitrogen as a gas. The outcome is a very basic alkyl anion. The aqueous medium protonated it immediately to make the final product.
Wolff-Kishner Reduction examples; Wolff-Kishner reduction, which applies to aromatic hydrocarbons, is exemplified by reducing cyclohexanone to cyclohexane. This is an example of the process.
Applications of the Wolff-Kishner Reduction
A pre-formed hydrazone can offer advantages, like decreased reaction time, room temperature reactions, or very mild reaction conditions, in comparison to the condensing of hydrazine to produce a hydrazone. The Wolff-Kishner reduction has numerous applications. Some of them are:
- Organic production of carbon nanotubes with many walls
- Functionalized imidazole substrates
Conclusion
Wolff-Kishner reduction is a chemical process that removes a carbonyl group from an alkane. The appropriate alkane can be produced by reacting these hydrazones with base and heat. The process produces methylene groups from carbonyl groups. Wolff Kishner reduction for ketones uses hydrazine (NH2NH2) as the catalyst in the presence of a strong base (KOH) in a protic solvent with a high boiling point. The reaction is driven by the transformation of hydrazine into nitrogen gas. It takes almost 200°C to get the reaction going reasonably.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




