Willgerodt reaction, also known as the Willgerodt rearrangement or Willgerodt reaction, is a chemical reaction that uses ammonium polysulfide to produce an amide from an aryl alkyl ketone, alkyne, or alkene. The resulting carboxylic acid is formed as a byproduct of the main process. Alkyl chains with n between zero and five often undergo several reactions, with the amide group at the end.
Amino acids can be synthesised via the Willgerodt Reaction, which uses aryl ketone as the starting point. Thio-substituted iminium-aziridinium rearrangements generate an enamine, and the carbonyl group migrates to the end of the chain via a chain of thio-substituted iminium rearrangements.
Willgerodt–Kindler Reaction
In order to make relevant biological probes, thioamides and similar structures are becoming increasingly important. There have been a variety of ways to synthesise thioamides in the literature for a long time.
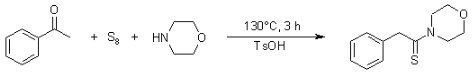
The Willgerodt-Kindler (WK) reaction, involving an aldone 1, cyclooctasulfur (i.e. cyclo-S8) and a primary or secondary amine 2, is a long-established one-pot reaction (first reported by Kindler in 1923), but it has received a lot of attention in the last ten years from the point of view of combinatorial chemistry. Even though it’s an old reaction, it’s revived interest due to the ease with which it can be used to introduce a variety of chemical diversity around the C(=S)–NH backbone by changing the aldone component R1 or amine component R2 or R3.
A large range of primary/secondary amines and aldones are now commercially available. Therefore this approach has the potential to generate a variety of pharmacologically relevant thioamides in one step. It is also worth noting that the WK reaction allows for a type of “umpolung” in which the carbonyl is reduced while the terminal methyl group is oxidised, starting with acetophenone, as an example.
Sulphur element and an amine, such as morpholine, are used in the similar Willgerodt–Kindler reaction. Thioacetamides, such as those from acetophenone, can be hydrolysed to the amide again.
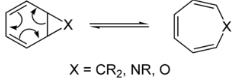
Pericyclic Rearrangement
Periodic reactions are those that take place when electrons change in the same direction again and over again (cyclically). This description outlines two different properties of a pericyclic reaction, both of which are discussed further below. What is immediately noticeable is that everyone responds in the same manner.
In a concerted reaction, there are no intermediates, which implies that the bonds between the reactant and the product are broken at the same time as the bonds between them. During pericyclic processes, the cyclic shift of electrons represents the second critical point.
Periodic motion is denoted by the phrase “pericyclic.” Pericyclic reactions are formed as a result of the cyclic shifting of electrons. Thus, pericyclic processes are characterised by the presence of an in-phase transition state that involves the pi bond.
Beckmann Rearrangement
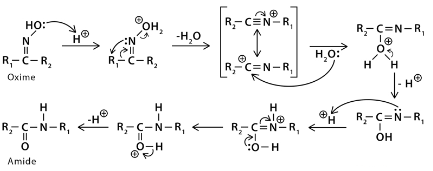
In the Beckmann rearrangement, an oxime is converted to an amide through the process of rearrangement. Oximes can be made by combining hydroxylamine with aldehydes or ketones. Lactams are synthesised from cyclic oxides by this rearrangement. NH groups can be inserted between the carbonyl carbon and alpha-carbon via the Beckmann rearrangement.
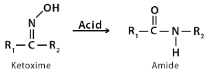
Large-scale cyclohexanone oxime rearrangement occurs in major industries because the product caprolactam is a direct predecessor to nylon 6, which is useful in a variety of applications like carpet and textile production.
Beckmann’s Rearrangement: Mechanism and Application
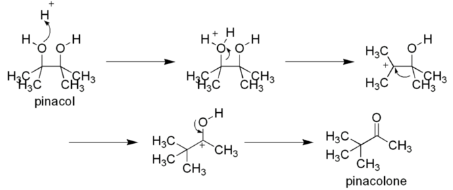
The Beckmann rearrangement follows the same pattern as a pinacol reaction in terms of its mechanics. The oxime OH is converted into a leaving group by the acid group present. The alkyl group, on the other hand, migrates to the nitrogen atom when the water is removed. An amide is formed when a cation and the water that was removed react.
Hoffman Rearrangement
The Hofmann Rearrangement is the outcome of bromine and hydroxide ion with water treatment of primary amide. As a result, an amine with no carbonyl group is formed. As a result, this rearrangement contributes to a one-atom reduction in the carbon chain. It also results in a switch from an amide to an amine functional group. Hofmann rearrangement has a very simple mechanism behind it. Sodium hydroxide and bromine are used to treat an amide. It’s an intermediate isocyanate that’s created when they are combined. The isocyanate loses a carbon dioxide molecule when water is added to the mixture, resulting in the amine molecule.
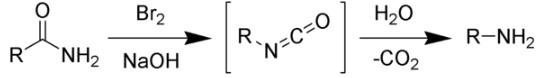
Conclusion
A series of 2-amino-1-phenyl-2-thioxoethanones have been synthesised using the condensation of haloacetophenone, Octasulfur, and Morpholine, with special attention paid to the atomic economy and efficiency of the synthesis process. Methods for making 2-bromo-1-phenylethanone and DMF in one pot have been developed. While most WK methods require high temperatures, this synthetic approach advances with good yields while only requiring a single room temperature. When Hendrickson wrote in 1975 about what he called an ideal synthesis, it involved just construction reactions, with no intermediary functionality, leading directly to the target. When it comes to optimal syntheses, the application of WK reaction to haloacetophenones is a noteworthy example.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out