Stobbe, in 1893, proved that the expected acetoacetic ester condensation to yield beta-diketo compound, but did not occur when a mixture of acetone and diethyl succinate was treated with sodium ethoxide. Stobbe condensation is the reaction of aldehydes or ketones with an ester of succinic acid to produce alkylidene succinic acid or isomers generated by a tautomeric transfer of hydrogen. The primary product is the salt of the half-ester, which requires one mole of metal alkoxide per mole of carbonyl chemical and ester.
Condensation reactions come in a wide variety of forms. For example, a typical example is the aldol condensation and the Knoevengel condensation, both of which produce water in their by-products, and Claisen and Dieckman condensations, which produce alcohols. Intramolecular condensation happens when atoms or groups of atoms in the same molecular structure come together, resulting in the production of rings.
Condensation Process
When two molecules or moieties (functional groups) join together to form a larger molecule, a smaller molecule is lost in the condensation reaction, also known as dehydration synthesis. When tiny molecules are lost in biological reactions, the most common is water, followed by hydrogen chloride, methanol, and acetic acid.
Carbonyl and carboxylic ester groups are the most significant activating groups in chemical synthesis. The enolate anion stabilises the resonance of the corresponding -carbanion when a proton is removed from the -carbon atom of a carbonyl compound with a base. These enolate ions play a key role in base-catalysed reactions of carbonyl compounds. The Stobbe reaction between dialkyl succinate and an aldehyde or a ketone is suitable for forming carbon-carbon bonds among various base-catalysed reactions. Benzoin condensation, in which aromatic aldehydes (without – hydrogens) are condensed with CN to produce C-C bonds, is another essential reaction for their synthesis.
Process of Stobbe Condensation
In the presence of a basic catalyst like sodium hydroxide or potassium tertiary butoxide, the reaction of aldehyde or ketone with the succinic ester to create alkylidene succinic acid is known as Stobbe condensation. Although the carbonyl compound can be altered over a wide range, the reaction is unique for succinic esters.
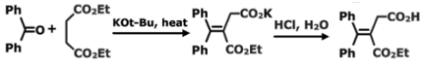
Aldehydes, on the other hand, are more commonly used than ketones. Acidification is then used to extract the product from the reaction mixture, including ketone, diethyl succinate and a sodium ethoxide solution. When potassium tertiary butoxide and tertiary butyl alcohol are used as bases, the yield and reaction time improve.
A lactone intermediate is used in the reaction to form salts of unsaturated esters, which are then removed by base catalysed elimination, causing an essentially irreversible opening of the ring. A lactone intermediate has been identified and isolated to support the mechanism.
Esters with only one hydrogen can’t undergo irreversible ring-opening in the final step, which drives the reaction forward. Only the lactone intermediate has been identified in the case of these esters.
Applications
A variety of unsaturated and saturated acids have been synthesised via Stobbe condensation. Polycyclic ring structures can be synthesised with the condensation process as well. It has also been employed during the production of estrone. A basic catalyst, such as sodium hydroxide or potassium tertiary butoxide, is required for the reaction of aldehyde or ketone with the succinic ester to produce alkylidene succinic acid.
The Darzens Condensation
Darzens halogenation produces alkyl halos from alcohols using a tiny amount of nitrogen base (such as trimethylamine or trimethylpyridine) and a significant amount of thionyl chloride or trimethyl bromide (SOX2). In the presence of a base, the Darzens Reaction results in the formation of an-epoxy ester via condensation of a carbonyl molecule with a -halo ester.
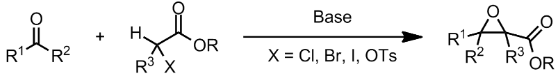
R−OH + SOCl2 + pyridine → RCl + SO2 + HCl
Conclusion
When two molecules join to produce a single molecule in organic chemistry, it is called a condensation reaction. This type of chemical reaction typically results in the loss of a tiny molecule, such as water. Dehydration synthesis refers to a reaction in which water is lost. Ammonia, ethanol, acetic acid, and hydrogen sulphide are just a few of the many chemicals that might be lost in the process.
As a basic catalyst, sodium hydroxide or potassium tertiary butoxide can be used to react aldehyde or ketone with the succinic ester to produce alkylidene succinic acid. Diester ester groups at the first and last carbons are the only ones that can be used in the Stobbe reaction. There are many different types of reactions that can occur, depending on the acidic or basic circumstances and the presence or absence of a catalyst. This family of reactions is fundamental to creating peptide bonds between amino acids and the production of fatty acids.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






