The Michael reaction converts a ketone or aldehyde to a carbonyl molecule by pulling it apart. The addition of a double-stabilised carbon nucleophile to an unsaturated carbonyl compound, such as malonates, cyano esters, and ketoesters, which are all nucleophiles. An extremely valuable 1,5-deoxygenated pattern is present in the final product.
Michael acceptor diethyl malonate reacts with diethyl fumarate, diethyl malonate and mesityl oxide, diethyl malonate and methyl crotonate, diethyl malonate and 2-nitropropane, methyl vinyl ketone, nitropropane, 2-nitropropane, methyl vinyl ketone and ethyl phenyl cyanoacetate
The Michael addition is a crucial atom-efficient method for the formation of C–C bonds. The Robinson annulation is a classic Michael and aldol addition tandem.
History
Conrad and Kuthzeit publication about the reaction of ethyl 2,3-dibromopropionate with diethyl sodiomalonate in 1884 led to Arthur Michael’s research at Tufts University in 1887.
With 2-bromacrylic acid ethyl ester in place of propionate, Michael discovered that the reaction could only function by introducing the acrylic acid double bond. He generated the first Michael adduct using diethyl malonate and cinnamic acid ethyl ester.
Rainer Ludwig Claisen claimed invention priority. In 1883, he and T. Komnenos discovered double bond addition products as side products of malonic acid condensation. A biographer, Takashi Tokoroyama, says that this claim has no basis.
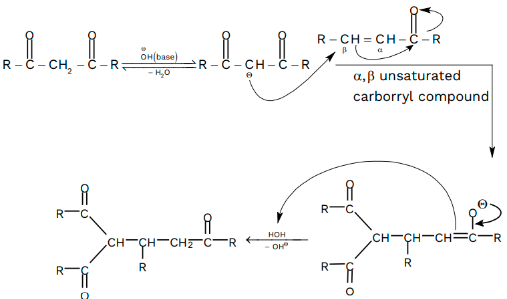
Michael Addition Mechanism
A Michael donor is a nucleophile or base that donates an electron to a proton. The non-bonding electrons in acyl and cyano groups are ready to donate energy, making them efficient nucleophiles. The base or nucleophile abstracts the acidic hydrogen attached to the substrate, methane, to produce the carbanion. The carbanion-forming substrate is the Michael donor, while the other substrate attacked by the donor is the Michael acceptor.
The reaction is thermodynamically regulated, and the result is thermodynamically stable. Most Michael donors are active methylene, with electron-drawing groups attached to the carbon proton. The electron withdrawing group attached to the neighbouring carbon makes abstracted protons very acidic. Michael acceptors are usually electron-deficient olefins.
Michael Addition Mechanism Steps
Step 1
The base deprotonates – hydrogen, forming carbanion in the first step. Because oxygen has a more stable negative charge than carbon, carbonyl carbon stabilises the negative charge on carbon through resonance.
Step 2
The electron-deficient Michael acceptor accepts electrons from the electron-rich carbanion in the second step. As a result of their reaction, it forms a carbon-carbon bond. Carbon-carbon bonds are more stable than carbon-oxygen bonds, even though oxygen’s negative charge is more stable in the resonance structure.
Step 3
The carbonyl molecule accepts an electron from the solvent in the third step.
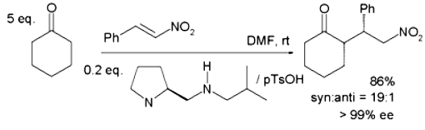
Asymmetric Michael Reaction
Recent studies have focused on asymmetric Michael additions. Chiral phase transfer and organocatalysis use chiral quaternary ammonium ions from Cinchona alkaloids.
Proline derivatives using cyclohexanone and nitrostyrene and acts with protic acids like p-toluenesulfonic acid: 95% of ee favour syn addition. Protonated amines in the proline side group are hydrogen-bonded to the nitro group in the enamine in the transition state, which is responsible for this selectivity. The nitro group generates hydrogen bonds with the proline side group’s protonated amines.
Michael Reaction Applications
The Michael addition reaction is well-known in organic synthesis. We commonly use it in the production of natural products and medications. People’s environmental consciousness has grown, and green chemistry research has advanced since the beginning of the 21st century.
What is Mukaiyama-Michael Addition?
The Mukaiyama–Michael reaction is a powerful method for generating two chiral centres on acceptor and donor components. With alkylidene malonates, Evans made 2,3-disubstituted tertbutyl 4,4-dicarbomethoxy-butanethioate. The diastereoselectivity depends on the enol silane geometry. Compared to the level of achiral Michael donor used, the enantioselectivity has a lot of space for improvement.
Michael Donors and Michael Acceptors
In Michael addition reactions, we call nucleophiles “Michael donors.” On Michael donors, the substituent groups are usually electron-withdrawing. The activated unsaturated compound’s substituent groups are Michael acceptors. Michael acceptors include ketone and nitro groups.
What is Michael’s addition mechanism’s contribution to organic chemistry?
It is a term that refers to the conjugate addition of a carbanion to an enal, enone, or other α, β-unsaturated compound. It creates a new carbon-carbon bond at the α, β-carbon. The nucleophile in the Michael reaction is the donor.
Conclusion
Michael addition is a well-known process in organic synthesis. Adding a carbon nucleophile to an unsaturated carbonyl molecule forms a carbon-carbon bond.
The Michael reaction consists of adding a carbanion to an α, β-unsaturated carbonyl molecule that contains an electron withdrawing group. When it comes to C–C bonds, conjugate additions are among the most common methods for making them. There are numerous asymmetric variants.
The nucleophile (Michael donor) draws electrons from the neighbouring methylene hydrogen, making it acidic enough to form a carbanion when reacting with the base, B. The alkene R might be a ketone, nitro group, or sulfonyl methyl fluoride (the Michael acceptor). It results in an enone. It is the Michael adduct.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



