The Lossen rearrangement is a transformation in which a hydroxamate ester is converted into an isocyanate. The O-acyl, O-sulfonyl, and O-phosphoryl derivatives are the most often encountered. When isocyanate is combined with certain amines or when the isocyanate is combined with water, urea is formed.
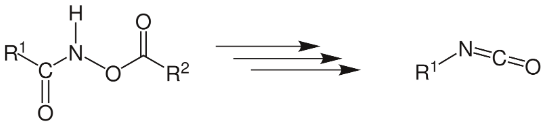
Mechanism of Reaction
When H2O is present, an O-acylated hydroxamic acid derivative is treated with a base to form an isocyanate, which then reacts with an amine to produce CO2 gas and an amine byproduct. The base abstraction of hydrogen from the hydroxic acid derivative is the first step in transforming it into its conjugate base.
In the presence of a carboxylate anion, the isocyanate intermediate is formed by spontaneous rearrangement. The hydrolysis of the isocyanate occurs in the presence of water, which causes the chemical to degrade and decompose. Lastly, decarboxylation and proton abstraction reactions are employed to yield the appropriate amine and CO2.
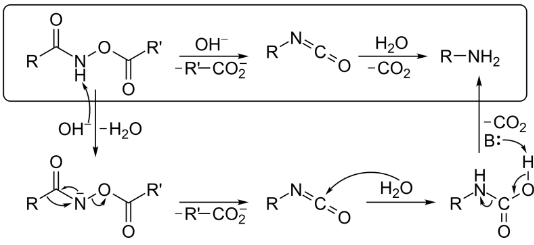
Under Neutral Conditions
The Lossen rearrangement can be carried out under neutral conditions without the need to treat the hydroxamic acids in advance, which is a beneficial variant of the original procedure. An amine is produced as a result of the Lossen rearrangement, which is achieved by briefly heating hydroxamic acid in formamide. With the synthesis of aminopyridines and benzimidazole-2-ones, anthranilic hydroxamic acid and pyridine carbon hydroxamic acid are rearranged to provide the corresponding compounds.
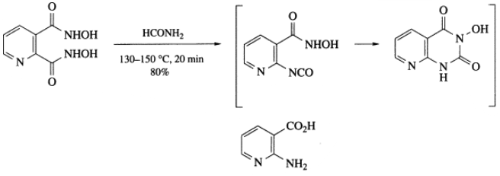
The Lossen rearrangement of hydroxamic acid, formed from quinolinic acid, occurs regioselectively and results in the formation of 2-aminonicotinic acid in high yield, first via the isocyanate and subsequently via the Leuchs anhydride-type intermediate. The latter intermediate is obtained by reacting benzenesulfonyl chloride with the disodium salt.
In the presence of formamide, four quinolinecarboxylic hydroxamic acids can be heated rapidly to make 4-aminoquinoline via the isocyanate, whereas increasing the temperature slowly results in the formation of N-(4-quinolyl) urea. As previously mentioned, the intermediacy of O-for-mates or equivalents has been proposed as a possible modification of the Lossen rearrangement.
In the presence of neutral conditions, N,N’-dicyclohexylcarbodiimide (DCC) acts as a catalyst in the Lossen rearrangement of hydroxamic acids. During the Lossen rearrangement, the hydroxamic acid is added intramolecularly to the tricyclic molecule, resulting in a cycloaddition reaction.
The Mitsunobu reagent, a mixture of triphenylphosphine and diethyl azodicarboxylate is used in the Lossen rearrangement to create O-(N-aryl carbonyl)-hydroxamates from aromatic hydroxamic acids, using the Mitsunobu reagent which is a mixture of triphenylphosphine and diethyl azodicarboxylate. A second spontaneous Lossen rearrangement may result in the formation of diaryl urea. The yields are 70 to 85 percent of their respective values. The phosphonium salts have the potential to act as a medium.
Under Acidic Conditions
In the presence of hydroxylamine or nitromethane, polyphosphoric acid interacts with aromatic carboxylic acids to produce aromatic amines. The intermediates are hydroxamic acids that have been activated by phosphorylation with polyphosphoric acid, and have then gone through the rearrangement step of the reaction.
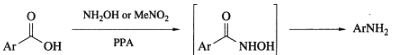
When nitromethane is used, it creates hydroxylamine as a byproduct of the polyphosphoric acid reaction that happens as a result of the polyphosphoric acid reaction. Aromatic acids carrying electron-donating substituents perform well in this one-step Lossen rearrangement. However, aliphatic acids and aromatic acids containing electron-withdrawing substituents perform poorly or generate insufficient yields in this reaction.
When using hydroxylamine-O-sulfonic acid, the Lossen rearrangement can be carried out successfully. It is necessary to improve the reaction conditions when heating acids in hot mineral oil or polyphosphoric acid to make amines, yet, this procedure has already produced amines.
Conclusion
The Lossen rearrangement, which permits hydroxamic acids to be transformed into isocyanates, was discovered nearly 150 years ago by chemists. A century has passed since the discovery that the dehydration of primary hydroxamic acids might be hastened by the presence of high quantities of activating reagents at stoichiometric ratios.
It has recently been demonstrated that the Lossen rearrangement can occur directly from free hydroxamic acids, causing a resurgence of interest in the process. With a strong emphasis on their mechanisms, this brief overview summarises achievements in this field by detailing progressively the metal-assisted, the spontaneous, and the promoted self-propagative Lossen rearrangements, with a particular emphasis on their mechanisms.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






