Aromatic compounds are arranged in the conjugated planar ring systems. They do not have alternating single and double bonds; instead, they have delocalised pi-electron clouds around them. They are also known as mono and polycyclic aromatic hydrocarbons. Aromatic compounds are derived from benzene and are used on a large scale in chemical industries and laboratories. Classified mainly as Benzenoids and Non-Benzenoids, these compounds can also be called aromatics or arenes. The chief examples under this category can be toluene and benzene. They need to satisfy Huckel’s rule in order to pass as aromatic compounds.
What are Aromatic Compounds?
Aromatic compounds are nonpolar compounds that have delocalised pi-electrons around them. They are non-miscible in water and have a sooty yellow flame. Most of the organic compounds are obtained by plants, microorganisms and animals. The hydrocarbons have sigma bonds. The aromatic compounds are called so because of their aroma.
Uses of Aromatic Compounds
Aromatic compounds have many uses in different industries. Some of the uses are as follows: toluene is widely used as a solvent for model glues, and naphthalene is used as mothballs to keep moths away from clothes.
They are also used in the manufacturing of explosives, drugs, and dyes. 1, 2 benzenediols or pyrocatechol is used as catechol and is an important part of photographic development.
Classifications of the Aromatic Compounds
Aromatic compounds can be majorly classified into two categories: Benzenoids and Non-Benzenoids. Both of them have their own dissimilarities and are quite distinctive in their characteristics. The Benzenoids contain benzene rings, whereas the Non-Benzenoids do not contain a benzene ring. The hydrocarbons that satisfy Huckel’s rule are called aromatic compounds.
- Benzenoids – Benzenoids are organic compounds with at least one benzene ring in their structure. The resonance in the benzene rings adds to the stability of the organic compound and makes them ineffective to changes, until and unless favourable conditions are met. They have three pi and three sigma bonds arranged alternatively inside the benzene ring structure. This pattern is also called the conjugated pi system. Toluene is a fine example of Benzenoids.
- Non-Benzenoids – Non-Benzenoids are organic compounds that do not have a benzene ring. They are devoid of any kind of benzene rings and have a different structure. They have a conjugated pi system, irrespective of the absence of the benzene rings. They contain 5 – 7 carbon atoms in the ring structures. The conjugated pi system imparts the aromatic nature to these compounds. Some most common examples are azulenes, oxazolidinones, pentafulvene, etc.
Huckel’s Rule
Huckel’s rule is used to determine the aromaticity of organic compounds. A cyclic molecule having ring shapr is said to follow Huckel’s rule when the sum of the pi electrons in the molecule satisfies the formula ‘4n + 2’. N stands for any positive integer. The value can be zero as well.
The molecules that can be taken as examples have the value of n between 0 to 6. The total number of pie electrons in the benzene molecule is 6. Have a look at the image of the benzene ring here. It obeys the 4n+2 𝛑 electron rule and n=1.
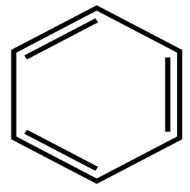
Thus, the benzene ring is aromatic in nature (because it satisfies Huckel’s rule).
The Pariser-Parr-Pople method is an important method that can be used to justify Huckel’s rule. The Pariser-Parr-Pople method is a method that provides a quantitative prediction of the electronic structures, and also spectra.
Conditions for Compounds to be Aromatic
There are certain conditions that need to be met in order to be an aromatic organic compound. They are given below:
- The molecule must exhibit a cyclic structure and nature and must definitely have a ring of p orbitals. Also, it gets fulfilled when the p orbitals do not have sp3 hybridisation.
- The system of connected p orbitals must have 4n + 2 𝛑 electrons. This condition needs to be met as the formation of aromatic compounds would not take place without this condition.
- The molecules are required to have a planar structure to be aromatic compounds. The p orbitals must interact with each other in parallel.
Conclusion
The aromatic compounds have a high resonance (especially in the case of Benzenoids) due to the presence of benzene ring(s). They can be broadly classified into Benzenoids and Non-Benzenoids. Examples of aromatic compounds include Aspirin and Paracetamol, which are medicinal drugs used in medical industries on a large scale. Chloroquine, the drug used to treat malaria, is also an aromatic compound. Huckel’s rule plays an important role in deciding whether the compound is aromatic or not.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






