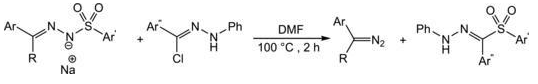
Researchers have discovered members of a class of N-arylsulfonyl hydrazones that work as potent inhibitors of IMP-1, a type of Metallo β—lactamase that becomes highly prevalent. Studies of the structure-activity connection have revealed that bulky aromatic substituents on either side of the sulfonyl hydrazine chain are required for these compounds to be effective inhibitors of IMP-1. The structural foundation for the anti-Metallo β—lactamase activity demonstrated by this family of drugs has been discovered by molecular modelling. Easily prepared from carbonyl compounds, hydrazonoyl chlorides with sodium arylsulfonyl hydrazones are used to synthesise arylsulfonyl hydrazines in DMF.
Arylsulfonyl Hydrazone Salts
In chemical synthesis, N-arylsulfonyl hydrazones served as stable diazo surrogates. Among these, N-tosyl hydrazones occupied a central position in the investigation of carbene chemistry, with numerous reactions involving N-tosyl hydrazones and published reviews. Electron-withdrawing group (EWG)-substituted N-arylsulfonyl hydrazones have been rapidly produced as milder diazo intermediaries in recent years. The review summarises the current developments in research into EWG-substituted N-arylsulfonyl hydrazones, which are used as diazo intermediaries in organic synthesis reactions. It also emphasises the coupling, cyclisation, incorporation, multicomponent, and Doyle-Kirmse reactions that involve N -o- nitrobenzene-sulfonyl hydrazides and N-o-nitrotriftosyl hydrazones.
Compounds of the Aryl Sulfonyl Hydrazine Group:
N-arylsulfonyl hydrazones, a subgroup of acylhydrazones, play a crucial activity in medicinal chemistry. Biologically, they have a variety of functions, including antitumor, antibacterial properties, antiviral, and anti-inflammatory properties. They also operate as new inhibitors of the enzyme IMP-1 or carbonic anhydrase.
It has been discovered how to prepare sulfonyl hydrazides by oxidising N-alkyl sulfonamides in the presence of iodine. Several arylsulfonyl hydrazide compounds have been researched as antioxidants, anti-corrosion, and antiwear additives. Those sulfonyl hydrazides also have a broad spectrum of antibacterial and antifungal properties.
Preparation of Arylsulfonyl Compounds through Catalysis
N-arylsulfonylhydrazones are synthesised similarly to N-acylhydrazones. Under reflux, aryl sulfonyl hydrazines are reacted with the aryl/alkyl aldehydes or ketones in Brnsted-Lowry acid catalysts and protic polar solvents. The duration of the process ranges from minutes to hours. So, the frequent formation of a mix of E/Z diastereomers, although waste liquid contamination with poor stereoselectivity, is inevitable under these conditions.
Two primary categories of aryl sulfonation methodologies have been established in the last five years. The first involves direct arylsulfonyl action, and the second arylsulfonyl action involves several components. In the direct aryl sulfonation process, arylsulfonyl reagents undergo coupling or adding reactions with C–H Chemically activated molecules, alkynes, and alkenes. In multicomponent reactions, various arylation reagents and substrates react with sulphur dioxide surrogates like DABSO, Na2S2O5, and K2S2O5. Sulphur dioxide surrogates can be used to form an aryl sulfonyl source by reacting with sulfuric acid, and subsequent reactions are similar to those of a direct arylsulfonyl nation. Aryl Hydrazines, aryl boronic acids, aryl silanes and aryl halides are the most commonly used arylation reagents.
An amino group is formed by nucleophilically substituting benzyl mercaptide for one of the nitrogens in the respective nitroaromatic compounds. The benzyl sulphide group was then persulfonylated using 4-toluenesulfonyl chloride.
Inhibitor Synthesis
It has been discovered how to prepare sulfonyl hydrazides by oxidising N-alkyl sulfonamides as in the existence of iodine. Some arylsulfonyl hydrazide compounds have been investigated as antiwear, antirust, and oxidation resistance additives in various applications. Additionally, these sulfonyl hydrazides have strong antibacterial and antifungal properties.
Most of the time, sulfonyl chlorides are used to add a sulfonyl protecting group. They can be transformed into many different sulfonyl derivatives, get through the different desulfitative cross-couplings, and act as acylating agents. Elastomers, medicines, dyes, detergents, and ion exchange resins are all products that rely on the use of these building blocks. In addition, they have recently shown importance in synthesising receptors and catalysts. Because of their widespread application in various sectors, there is a great deal of interest in preparing a more efficient synthetic process for extracting them. One of the most common synthetic methods is multiple oxidising agents and chloride sources to oxidise the thiols.
Sulfonyl hydrazides are desirable targets due to their wide range of uses in organic synthesis, namely total synthesis. Furthermore, due to the high reactivity of sulfonyl chloride, several sulfonyl hydrazides could be synthesised by reacting them with hydrazine hydrate.
(i) Procedure for Preparing Arylsulfonyl Hydrazides in General
A solution of the suitable sulfonyl chloride (2.5 mmol) was added over 2 minutes to the solution of anhydrous hydrazine (12.5 mmol) in CH2Cl2 (5 ml) that was being stirred. After 15 minutes of stirring, the pH of the reaction solution was changed to approximately 11 using 10 percent NaCO3. Then, separating the layers and extracting the aqueous phase with CH2Cl2. The mixed organic compounds were dried over MgSO4, filtered, and then the solvent was extracted at reduced pressure. Therefore, there was no need for additional purification of the product.
(ii) Procedure for Preparing Arylsulfonyl Hydrazones in General
In hot ethyl alcohol, 0.5 mmol of the needed sulfonyl hydrazide and 0.5 mmol of the suitable aldehyde were mixed (2 ml). The result was precipitated by adding water dropwise after the mixture had cooled to room temperature. After the precipitate was filtered, it was washed with 2 ml of cold H2O and 2 ml of hexane and then dried in a vacuum.
Conclusion
The unique anti-HIV, anti-cancer, and antibacterial bioactivities of sulfone-containing chemicals make their synthesis a major focus in pharmaceutical and agrochemical research. On the other hand, Sulfones can be used in chemical synthesis as versatile building blocks. Organic molecules have increasingly been synthesised with sulfonyl hydrazides, frequently used for this purpose over the last few decades. For these reactions, the production of carbonyl carbocation in the carbonyl group is determined by electronic and steric variables, both of which play an important role. This method is practical and appealing for the synthesis of aryl sulfonyl hydrazones. In addition, antimicrobial screening investigations were carried out. The findings indicate that a number of the chemicals had effective antibacterial properties.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






