DMSO/DCC Pfitzner–Moffatt oxidation uses a combination of dimethyl sulfoxide (DMSO) and dicyclohexylcarbodiimide (DCC) in the presence of a proton source to convert primary and secondary alcohols to their corresponding aldehydes and ketones under mild and nearly neutral conditions. The reaction applies to primary and secondary hydroxyl groups in a wide range of compounds. This reaction involves DCC activating DMSO via an acid-catalysed process.
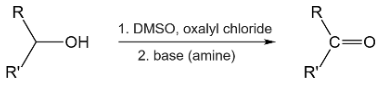
The activator for dimethyl sulfoxide (DMSO) in the DMSO/DCC Pfitzner swern variant is dicyclohexylcarbodiimide (DCCI). After the DMSO attacks the carbodiimide, the added alcohol attacks the positively charged sulphur atom. The molecule becomes oxidised into a carbonyl after alpha hydro-elimination. As an activator, dicyclohexylcarbodiimide (DCCI) may boost Pummerer rearrangement and reduce side reactions.
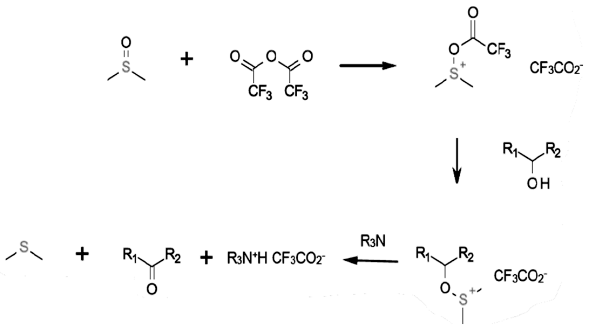
Trifluoroacetic anhydride and pyridine SO3 have mostly replaced DMSO/DCC Pfitzner. The moderately insoluble dicyclohexyl urea by-product typically precipitates from the solution once the reaction is complete. Filtration will not remove it since it is partially soluble. We must consider the dimethylsulfide by-product due to its toxicity and stench.
Pfitzner-Moffatt TFAA Activation Variation
This swern variant uses trifluoroacetic (TFAA) as the DMSO activator. The DMSO attacks the TFAA first, enabling the alcohol to attack the sulphur. The result of alpha hydroxy elimination is oxidation to a carbonyl.
Oxidation with DMSO
Combining with an activating agent and an amine base, DMSO is a moderate and selective reagent for converting alcohols to aldehydes and ketones. Aldehyde overoxidation isn’t a factor. Although Dess-Martin periodinane can be very selective, it can also cleave glycols and carbonyl compounds like other transition metal oxidants (Cr, Ru, Os, and Mn).
- Moffat discovered the oxidation by activating DMSO with acetic anhydride.
- This reaction describes several subsequent modifications that have improved the process.
- We can generate the active intermediate Me2S-X differently in each of these systems.
- Parikh-Doering, Pfitzner-Moffat, Carbodiimides + DMSO, Corey-Kim, and Swern are examples of this oxidation type.
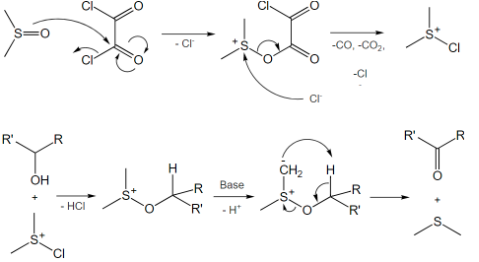
- Swern oxidation includes DMSO + oxalyl chloride or DMSO + trifluoroacetic anhydride.
- There are several methods for activating the DMSO, but Swern’s use of oxalyl chloride and triethylamine to activate DMSO is the most popular.
- We use the Kornblum oxidation less often, in which O-alkylation of DMSO generates an intermediate alkoxy sulfonium salt through an allyl or benzyl halide.
- Because we can use the reaction mixtures directly for subsequent transformations, we can even prepare aldehydes that are too unstable to isolate.
- It is challenging to isolate formyl silanes because of their instability. We use a Lithium reagent to trap an in-situ formed aldehyde.
- DMSO oxidants do not convert sulphides or amines to amine oxides, which can be problematic for the oxidation of alcohols with many other oxidants.
What is DMSO – Pyridine-SO3?
The Swern variant uses Trifluoroacetic anhydride and pyridine SO3 complex as an activator, similar to the Swern and Pfitzner-Moffatt mechanisms.
What are the Applications of DMSO?
DMSO is a non-toxic polar aprotic solvent that is less harmful than dimethylformamide and HMPA. We frequently use DMSO as a solvent in salt-based reactions, including Finkelstein reactions and nucleophilic substitutions. It’s a common extractant in cell biology and biochemistry. DMSO’s low acidity in carbanions research makes it a great option because it can tolerate relatively strong bases.
DMSO evaporates slowly under normal atmospheric pressure due to its high boiling point of 189°C (372°F). Samples dissolved in DMSO are more difficult to recover than those dissolved in other solvents because conventional rotary evaporation cannot remove all traces of DMSO. Then we add water to dissolve DMSO and cryodesiccation to remove both organic solvent and water. Water is frequently added to DMSO reactions to precipitate or separate products. Because of its high freezing point of 18.5°C, we often use DMSO as a solid at or below room temperature.
Deuterated DMSO (DMSO-d6) is a valuable solvent for NMR spectroscopy because it dissolves many analytes and has a simple spectrum. The disadvantage of using DMSO-d6 is the high hygroscopicity and viscosity, and DMSO-d6 causes an overwhelming H2O resonance in the 1H-NMR spectrum. We often mix it with CDCl3 to reduce viscosity and melting point.
High-throughput screening programmes also use DMSO to dissolve test compounds for in-vitro drug discovery and design.
A high boiling point and ability to dissolve both polar and nonpolar compounds make it excellent for the storage and analysis of chemical compounds. DMSO may affect cell growth and viability. Low DMSO concentrations can stimulate cell growth, while high concentrations inhibit or kill cells.
In-vivo studies of test substances also use DMSO. We use this co-solvent to help the nematode Caenorhabditis elegans absorb flavonol glycoside Icariin. DMSO’s use in animal models has the same limitations in in-vitro studies. Improper DMSO control groups may lead to misattribution of solvent effects to the prospective drug. For example, a very low dose of DMSO protects mice from paracetamol (acetaminophen) induced liver injury.
We are also increasingly using DMSO in microelectronic device manufacturing processes. We also use them for advanced packaging (including wafer-level/solder bump patterning) and photoresist stripping in TFT-LCD displays. Additionally, DMSO is a safe paint stripper compared to other compounds, like nitromethane and dichloromethane, making it a viable option.
Conclusion
We use the DMSO/DCC Pfitzner swern oxidation procedure to oxidise secondary alcohols. However, it is not an ecologically favourable process since it produces significant amounts of foul-smelling dimethylsulfide. The development of modified Swern oxidations creates odourless sulphides using polymer-bound dimethyl sulfoxide or long-chain sulfoxides. This has been the focus of significant research in recent years.
To create soluble polymer-bound oxidising reagents, sulfoxides bond to polyethylene glycol (PEG). These reagents are easily separated and recycled in a Swern procedure. Sulfoxides bound to non-crosslinked polystyrene (NCPS) are an additional instance of soluble polymer-bound sulfoxides. These reagents are also quickly isolated and recyclable and used successfully in the Swern oxidation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






