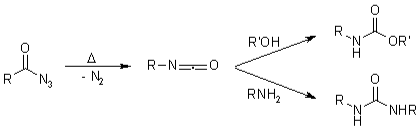
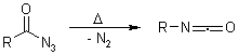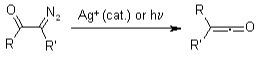The heat degradation of an acyl azide toward an isocyanate with the emission of nitrogen gas is also known as Curtius rearrangement. The isocyanate is subsequently attacked by nucleophiles like water, alcohols and amines, resulting in a primary amine, carbamate, or urea derivative. The transformation of a hydroxamate ester toward an isocyanate is known as the Lossen rearrangement. O-acyl, sulfonyl, and phosphoryl O-derivatives are often used. The isocyanate could also be used to make ureas there in the existence of amines. The Wolff rearrangement seems to be an organic chemistry process in which a -diazo carbonyl molecule is transformed to a ketene by dinitrogen loss and 1,2-rearrangement.
Curtius Rearrangement
Curtius reorganisation is also known as Curtius degradation. According to Theodor Curtius in 1885, the Curtius Rearrangement seems to be the heat breakdown of acyl acid to create isocyanate with the elimination of nitrogen. Curtius deterioration or Curtius response are other names for it. Schmidt Reaction is the same as this process.
Isocyanates were attacked by nucleophiles such as alcohols, water, and amines, resulting in the formation of urea derivatives, carbamate & essential amines.
Curtius Rearrangement Process
Preparation of Acyl Azide
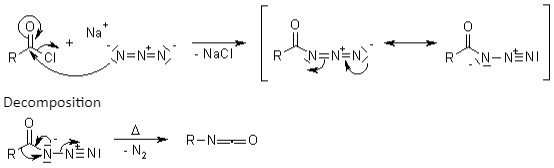
When it comes into contact with water, it forms a hazardous carbamic acid derivative. It also passes through spontaneous decarboxylation.
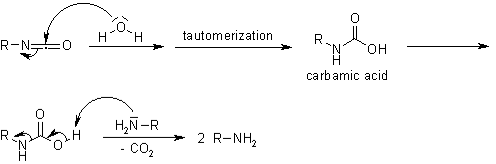
Isocyanates are indeed a versatile building block. Isocyanates have an important function in polymerisation & biomacromolecule derivatisation.
Wolff Rearrangement
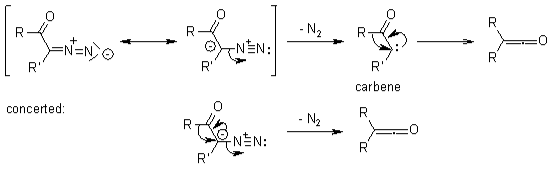
Ketenes may be made from -diazo ketones via the Wolff Rearrangement. Due to its high reactivity to produce diketenes, such ketenes were usually not separated.
Within the existence of nucleophiles, Wolff rearrangements produce carboxylic acid derivatives, but in the existence of unsaturated molecules, [2+2] cycloadditions could occur.
The creation of -diazo ketones from carboxylic acids, followed by the Wolff Rearrangement there in the existence of nucleophiles, outcomes in carboxylic acids being homologated with one carbon. Arndt & Eistert devised the chemical series, which was the first one to demonstrate the Wolf-synthetic Rearrangement’s potential.
Wolff Rearrangement’s Process
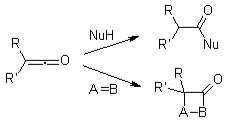
Since there are many conflicting coordinated and sequential processes, the mechanistic route of Wolff-rearrangement seems to be the topic of significant discussion. Two features of the system, though, may be concurred upon. Firstly, s-cis with s-trans-conformers are all in balance in -diazo carbonyl substances, and their distribution might affect the reaction process. According to the antiperiplanar interaction between both the departing and migratory groups, molecules inside the s-cis shape respond uniformly under photocatalysis, while those inside the s-trans shape react sequentially throughout a carbene intermediary or do not reorganise. Secondly, irrespective of the reaction process, the rearrangement produces a ketene intermediary, that can be captured by a slightly acidic nucleophile, like an ethanol or amino group, to produce the equivalent esters or amine groups, or perhaps an olefin to produce a cycloaddition compound. Acidic solutions do not reorganise the -carbon but instead protonate it and produce SN2.
Loosen Rearrangement
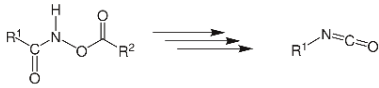
The transformation of something like a hydroxamate ester to that of an isocyanate is known as the Lossen rearrangement. O-acyl, sulfonyl, and phosphoryl O-derivatives are often used. The isocyanate could also be used to make ureas there in the existence of amines.
Loosen Rearrangement Process
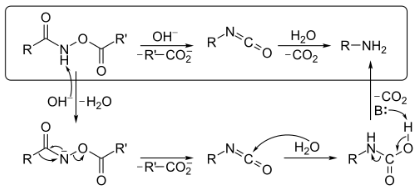
There in the existence of H2O, the O-acylated hydroxamic acid derivatives are handled using the base to make an isocyanate, which produces an amine but also CO2 gas. This hydroxamic acid derivative will be first transformed towards its conjugate base via extraction of something like hydrogen by a base. To form the isocyanate phase, spontaneously rearranging produces a carboxylate anion. There in the existence of H2O, this isocyanate would then be hydrolysed. Ultimately, through decarboxylation and extraction of protons with such a base, the corresponding amine & CO2 are produced. Hydroxamic acids were routinely made from their ester counterparts.
Conclusion
The Curtius rearrangement is the thermal breakdown of just an acyl azide to something like an isocyanate only with the emission of nitrogen, as initially characterised by Theodor Curtius around 1885. The isocyanate can subsequently be attacked by nucleophiles including water, ethyl alcohol, or amines, resulting in an amine group, carbaryl, or urea derivatives. The Wolff rearrangement seems to be a chemistry process during which a -diazo carbonyl molecule is transformed to a ketene via dinitrogen elimination with 1,2-rearrangement. This Wolff rearrangement produces a ketene like reaction product that may be nucleophilically attacked with slightly acidic nucleophiles including water, ethyl alcohol, or amines to produce carboxylic acid equivalents or [2+2] cycloaddition events to construct four-membered groups. The transition of a hydroxy ester to that of an isocyanate is known as the Lossen rearrangement. O-acyl, sulfonic, and phosphoryl O-derivatives are often used. The isocyanate could also be used to make macronutrients in the environment of amines or organic compounds in the case of moisture.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out