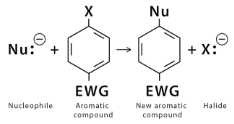
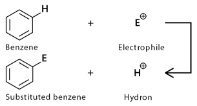
As the name suggests, substitution reactions are those reactions wherein a compound, one functional group is replaced by another functional group. Aromatic compounds are organic molecules that have 1 or more than 1 planar ring of atoms joined by single or double covalent bonds. The most common and basic aromatic compound is Benzene. The aromatic compound has a planar structure with 6 Carbons attached by covalent double and single bonds. The reactivity and orientation of the aromatic substitution reaction are greatly affected by the substituents used during the aromatic substitution reactions. Electron withdrawing groups and electron-donating groups are important when determining the orientation and reactivity of an aromatic compound.
Types of Aromatic Substitution Reaction
There are 2 types of aromatic substitution reactions namely;
- Nucleophilic Aromatic substitution reaction- this is a type of reaction where a nucleophile is a compound that replaces the good leaving group such as halide ion present on the aromatic ring. Nucleophilic aromatic substitution reactions are not very common because the aromatic rings are themselves nucleophilic most of the time.
- Electrophilic Aromatic substitution reaction- here the Hydrogen atom or any other atom of the aromatic ring is substituted by an electrophile. Common electrophilic substitution reactions are nitration reaction, Halogenation reaction, Sulfonation, Friedel-Crafts alkylation, and acylation reaction. The upcoming topics have been discussed based on electrophilic aromatic substitution reactions.
Orientation in Aromatic Substitution reaction
The most classic reaction studied for orientation in an aromatic substitution reaction is the nitration of benzene. During a nitration reaction when the Benzene ring is substituted with some other group it highly affects the orientation of the nitro group in the benzene ring. The position of the nitro group, that is whether it is ortho, para, or meta directing, is highly regulated by the substituted group present in the benzene ring. It also affects the reactivity of the compound formed. Some examples of the group affecting the orientation and reactivity of the compound
Substituent | Percentage Ortho | Percentage Para | Percentage Meta | Relative reactivity |
H group | – | – | – | 1 |
CH3 group | 56.5 | 3.5 | 40 | 24 |
C(CH3)2 | 12.0 | 8.5 | 79.5 | 15.7 |
CH2Cl | 32 | 15.5 | 52.5 | 0.302 |
Cl | 29.6 | 0.9 | 68.5 | 0.033 |
Br | 36.5 | 1.2 | 62.4 | 0.030 |
NO2 | 6.4 | 93.2 | 0.3 | Approx. 10-7 |
CO2C2H5 | 28.3 | 68.4 | 3.3 | 0.003 |
Therefore, different substitution groups in the aromatic compounds show different orientations and reactivity of the compounds. The orientation of the products of the Aromatic substitution reaction plays an important role in deciding the stability of the intermediates and the speed of the reaction.
Electronic Effects
For aromatic compounds, the substituent in the Ortho and para position of the aromatic ring have greater electronic influence when compared to the meta position. If the substituting group is an electron-withdrawing group, then it destabilizes the compound and if the substituting group is electron-donating then it stabilizes the compound.
Meta Directing Compounds
Any electron-withdrawing group that is substituted at the meta position in the benzene ring deactivates the ring. Groups that fall under this category are NO2, CF3, CO2R, and many more.
Ortho and Para Directing Compounds
Electron withdrawing groups substituted at Ortho and para positions during a reaction decrease the reactivity of the compound.
The presence of an Electron donating group at the Ortho and para positions stabilize the intermediate compound of the substitution reaction. Groups that function as electron-withdrawing groups are the OH group, OR group, NH2 group, and many more such groups.
Steric Effect
The reactivities of the compounds having groups being placed at Ortho and para positions are not equal. It is known that most aromatic substitution reactions favor Substitution at the para position. The simple reason behind this is, that any group at the Ortho position gives more steric hindrance between the substituent and the group.
Conclusion
Aromatic substitution reaction is a type of reaction that is greatly influenced by the substitution groups. The reactivity of the reaction and the orientation of groups during the reaction plays a very critical role to establish the rate of the reaction and its productivity. The different substitution reactions form different products where the groups have different orientations. The orientation of the substituted group plays a major role in the stability of the compound. These types of reactions are a key procedure to produce different chemicals and compounds in chemistry. These are widely used in pharmaceutical companies and different laboratories for the production of different chemicals.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out