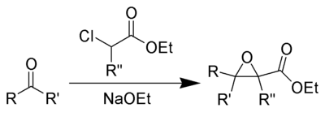
The Darzens glycidic ester reaction occurs when an aldehyde and a ketone combine with an α-halo ester to form an α, β-epoxy ester. It is alternatively referred to as a “glycidic ester.” Sodium ethoxide and sodium amide are the most commonly used condensing agents. The glycidic esters are significant because they can be transformed into aldehydes and ketones with higher saturation carbon content than the originating aldehydes or ketones. When an aldehyde is produced, this change occurs after the epoxy acid has been hydrolyzed and decarboxylated, along with a rearrangement.
The Chemical Reaction of Darzens Glycidic Esters
The Darzens reaction, alternatively referred to as the Darzens glycidic ester synthesis, is a chemical process in which a ketone or aldehyde reacts with α-halo ester in the existence of a base to generate an α,β-epoxy ester, also known as a “glycidic ester.” In 1904, the organic scientist Auguste Georges Darzens made the discovery known as the Darzens reaction.
The glycidic esters are significant because they can be transformed into aldehydes and ketones. This change occurs after the epoxy acids are hydrolyzed and decarboxylated and are followed by rearranging whenever an aldehyde is synthesised.
Mechanism of Reaction
The reaction occurs with the extraction of the ester’s acidic hydrogen. It then works as a nucleophile, reacting with the carbonyl molecule to generate a tetrahedral intermediate with the carbanion that has been formed. The following step involves intramolecular substitution reactions wherein the halide ion is removed to generate the epoxide.
The α-halo ester reacts with the carbonyl molecule to form syn and anti diastereomers.
The epoxide is then formed by an intramolecular S 2 reaction.
When the epoxide is formed, the cis: trans ratio is often between 1:1 and 1:2, which is typical. When it comes to aldehydes and ketones, the Darzens approach has traditionally been utilised as a homologation process without regard for stereocontrol, mostly in epoxide generation. Saponification of the α,β-epoxy ester, followed by decarboxylation, results in the formation of the substituted carbonyl molecule in this sequence.

Darzens’ method for constructing epoxides can also be utilised to synthesise -halo carbonyl compounds or similar compounds capable of deprotonation and containing electron-withdrawing groups. Additionally, the process can be performed using diazoacetate or a sulphur ylide as something like the leaving group.
The result of the reaction in the following substitution sequence is determined by the intensity of the addition, rotation, and ring closure transition.
As an alternative, Arai came up with an idea for a method for doing highly diastereoselective and enantioselective reactions.
The PTC recognizes one aldolate preferentially in this system. The equilibrium of syn- and anti-aldolates is established through retro-aldol addition, and the persistent, chelated lithium salt production prevents the subsequent uncatalyzed process from generating the epoxide product.
The underlying aza-Darzens process, in which a prepared lithium α-bromoenolate combines with a sulfinimine to generate an aziridine, exhibits a six-membered transition state, which contributes to the reaction’s high diastereoselectivity.
The advancement of enantioselective methods continues to be a challenging task. An aldol addition is an initial step in the Darzens Reaction; hence any strategy for stereoselective aldol additives can be tested.
Usage of Ester
The ester’s principal function is to facilitate the first deprotonation, and various carbonyl functional groups may be substituted. For example, when a -halo amide is used as the starting material, the result is an α,β-epoxy amide. The resulting compound is an epoxy ketone when α-halo ketone is employed.
The initial deprotonation can be carried out using any strong base. However, if the base material is an ester, the alkoxide matching to the ester side chain is frequently used to avoid problems caused by potential acyl substitution reactions.
Various Reactions
Darzens condensation can also be used to make glycidic esters, but it requires the preparation of the alkene substrates first. On the other hand, the nucleophilic epoxidation technique requires only one reaction to produce an alkene.
Successive Reactions
Many different compounds can be formed by further reacting to the Darzens reaction. For example, when the ester is hydrolyzed, it can result in decarboxylation, which causes the epoxide to be rearranged into a carbonyl. As a result, more structures can be formed through epoxide rearrangements.
Conclusion
The Darzens glycidic ester reaction occurs when an aldehyde or ketone is combined with an a-halo ester to form an α,β -epoxy ester (glycidic ester). The most common condensing agents are ethoxide and amide. Condensation of α-halo esters with carbonyl compounds in the presence of a base results in the formation of α,β-epoxy esters, which upon decarboxylation yield substituted carbonyl compounds.
An intramolecular nucleophilic substitution on the carbonyl carbon of the aldehyde or ketone is likely to be the mechanism for creating glycidic esters. Essential condensing agents have the role of converting the halo ester into its enolate counterpart.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






