Michael addition is the nucleophilic addition of a nucleophile (or a carbanion) to an unsaturated carbonyl molecule. It belongs to a group that is considered quite beneficial in the moderate creation of carbon-carbon bonds. Arthur Michael, an American chemical chemist, initially coined the term ‘Michael reaction’.
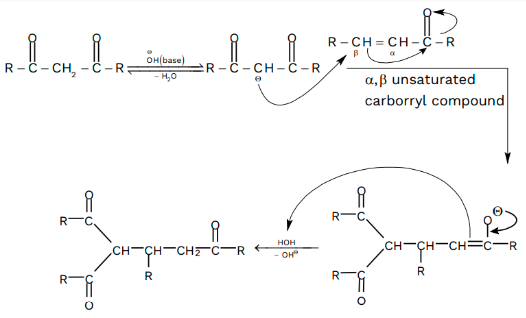
Starting with the deprotonation of the unsaturated carbonyl molecule, the base initiates the Michael addition process. This results in a carbanion, which is stable due to the electron-withdrawing groups it contains.
Key Steps in the Michael Addition Reaction
The nucleophile’s acyl and cyano groups are excellent substitutes for the nucleophile because they cause the methylene hydrogen to be acidic. This leads to the production of a carbanion when it combines with the base. The activated alkenes’ substituent group can either be a ketone or a nitro group. This substitute is known as a Michael acceptor.
First Step
In the first phase of the Michael addition mechanism, the base attacks the carbonyl-containing molecule. Afterwards, the carbonyl compound’s alpha hydrogen is deprotonated by the base, resulting in carbanion synthesis (which has enolate ions in two out of three resonance structures).
Second Step
To maintain its structure, the carbanion’s electron-withdrawing groups are responsible. A new carbon-carbon bond is formed when the enolate ion and Michael acceptor react together. As a result, the 1, 4 addition to the unsaturated carbonyl molecule is completed by the enolate ion.
Third Step
After the enolate has been added to the unsaturated carbonyl molecule, the protonated compound is generated. The mechanism of the Michael reaction is depicted here, along with the list of bonds that are produced and broken. Thus, a carbonyl compound’s enolate is added to an unsaturated carbonyl compound to produce the 1, 5 dicarbonyl compound.
Key Aspects
The demand for environmentally friendly and solvent-free processes has risen significantly in recent years. This organic transformation was made possible thanks to operationally easy catalytic procedures that avoid hazardous chemicals. To achieve desired transformations in a reasonable amount of time, solvent-free reactions sometimes call for a large catalyst loading.
The Michael reaction is one of the most important processes for forming carbon-carbon bonds. A wide range of Michael acceptors, including ketones, aldehydes, and esters, can be employed in this process to change into a wide range of functionality, such as nitriles.
Nucleophiles react with carbonyl compounds that are alpha and beta-unsaturated in the beta position in conjugate additions. Alpha, beta-unsaturated carbonyls can be synthesised for conjugate addition by halogenating carbonyls on their alpha carbons and then adding a weak base (often pyridine) to create a double bond. This method is widely used. An irreversible reaction takes place when strong bases (such as Grignard and organolithiums) assault the carbonyl group via 1, 2 addition. In a quick, but reversible, reaction, nucleophiles that are weaker bases are likely to react with the beta carbon through 1, 4 addition (conjugate addition).
Adding a carbanion to an alpha, beta-unsaturated carbonyl molecule causes the Michael reaction (also known as the Michael addition). A carbanion is added nucleophilically to an alpha, beta-unsaturated carbonyl in the Michael reaction.
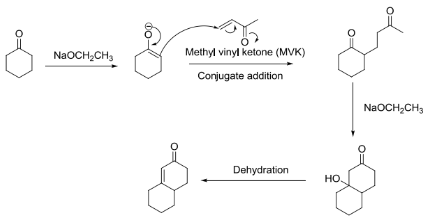
Robinson Annulation
Aldol condensation is then carried out intramolecularly in the Robinson annulation in order to generate the carbon-based cyclohexenone structure. The production of natural compounds, such as antibiotics, relies on the creation of cyclohexenone rings. Sir Robert Robinson, a chemist and 1947 Nobel laureate, is the inspiration behind the Robinson annulation. To obtain cyclohexenone via the Robinson annulation, a Michael reaction, enolate 1, 4-addition, and intramolecular aldol condensation are used.
Michael’s Asymmetrical Reaction
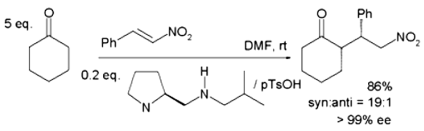
Asymmetric Michael additions (AMAs) have recently been the subject of research efforts to broaden their use. Organocatalysis, which uses enamine or iminium activation with chiral secondary amines, mainly produced from proline, is the most often used approach for chiral phase transfer catalysis.
When cyclohexanone reacts with nitrostyrene, a protic acid such as p-toluenesulfonic acid is used to derivatize the base proline. The enamine (produced between the proline nitrogen and cycloketone) and nitrostyrene are co-facial with the nitro group hydrogen bound to the protonated amine in the proline side group in the transition state considered to be responsible for this selectivity.
Conclusion
When a stable enolate attacks the beta carbon, the Michael reaction occurs. Both the Michael donor and the alpha,beta-unsaturated group are referred to as the Michael acceptor. The nucleophilic addition of a carbanion or another nucleophile to an unsaturated carbonyl molecule containing an electron-withdrawing group is known as the Michael reaction or addition. It is part of the conjugate addition family. This effective approach can achieve the mild production of C–C bonds.
A carbon-carbon bond is formed by the Michael addition. The Michael addition of acetylacetone to methyl acrylate is facilitated by a lipase variation. The Michael addition is an important reaction in organic synthesis because it leads to the formation of a carbon-carbon bond.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






