If you have been diagnosed with Malaria, you will often be prescribed antimalarials. Antimalarials are used to reduce the risk of Malaria. They can be given orally or by injection. These chemical agents may also be used to treat other illnesses, including rheumatoid arthritis, pneumocystis carinii pneumonia, arrhythmias (irregular heartbeats), and systemic lupus erythematosus.
Antimalarial medications include chloroquine (Plaquenil), quinine (Quinagra), and mefloquine (Lodrane). The list above is not exhaustive and more medications are being developed to fight this disease which kills thousands of people each year worldwide.
How do Antimalarials work?
These medications work in several ways. Some of them are effective because they kill parasites, some are effective because they prevent the parasite from infecting red blood cells, and some work both by killing the organism and making it harder for it to infect red blood cells.
What antimalarials work best?
The safest drug is the one that is cheapest and easiest to obtain. Chloroquine is excellent for most adults, but mefloquine is used for many people since it tends to have fewer side effects. It must be noted that although other chemical agents may be cheaper or easier to get hold of, they are not necessarily better than chloroquine or mefloquine.
How are Antimalarials consumed?
Antimalarials can be taken through oral or injection forms. Some people take their medications by injection, but this is not recommended for everyone since it can cause pain, swelling, and bloating when the drug is injected into your veins. Some people prefer to take their medications orally since this can reduce side effects and make antimalarial medicines more effective. That is why most people prefer to have their antimalarials in the form of tablets or capsules. In some cases, injections of the drugs may be more effective than oral administration.
Some common Antimalarials
Artemether/lumefantrine
It hinders the synthesis of nucleic acids and proteins by endoperoxide or possibly by hampering beta-hematin synthesis.
Artesunate
This drug has an iron endoperoxide bridge that generates oxidative stress. As a result, parasites are less likely to grow and survive since it restricts protein synthesis and nucleic acid synthesis.
Atovaquone
It blocks the exchange of electrons at the cytochrome BC1 complex which causes the collapse of the parasite mitochondrial membrane.
Atovaquone/proguanil
Atovaquone prevents electron exchange and collapses mitochondria, while proguanil inhibits dihydrofolate reductase, vital for parasite reproduction.
Chloroquine
It works by eliminating the erythrocytic form of Plasmodium, although its exact mechanism of action is still unclear.
Hydroxychloroquine sulphate
It is unknown exactly how it works against Plasmodium. The weak base may inhibit heme polymerization by affecting the acid vesicles of parasites. Other enzymes may also be involved.
Mefloquine
A structural analogue of quinine, mefloquine kills schizonts in the blood, though its exact mechanism is unknown. Intravesicular pH may be increased in parasites as a consequence.
List of conditions treated by Antimalarials
Apart from Malaria, Antimalarials are also used in conditions like
Pneumocystis carinii pneumonia
These medications help reduce the symptoms of this disease by helping to prevent further lung damage.
Arrhythmias (irregular heartbeats)
These chemical agents can help regulate heartbeats by making the heart muscles more healthy and reducing inconsistent behaviour such as fibrillation.
Rheumatoid arthritis
These chemical agents can reduce swelling, pain and tenderness in affected areas. They can also help prevent deformity of joints.
Systemic lupus erythematosus
These chemical agents make it easier for the body to fight off infections since lupus patients have an increased risk of acquiring infections.
Amebiasis, extraintestinal
Medications like quinine sulphate can be used to prevent infection of the liver, spleen and lungs since this disease is caused by amoeba.
Are there any side effects?
There are some side effects associated with antimalarials. Some symptoms may show up right away, but many of the more severe side effects do not show up until months or even years after taking the medication. The most common and reversible side effects include
Some Common Side Effects of Antimalarials | ||
|
|
|
Conclusion
Antimalarials are beneficial chemicals which can be used to treat or prevent Malaria. They are also used for other conditions like Pneumocystis carinii pneumonia and arrhythmias (irregular heartbeats). While there is still to be discovered about antimalarials, they are an essential medication that must be consumed and taken seriously. The chemicals of medical importance are all linked with a series of chemical reactions which cause the medications to work against parasites. Sometimes, these reactions can cause side effects, depending on various factors. Similarly, antimalarials also have some side effects.

Physical Properties of Aromatic Amines
Aromatic amines have their lone pair of electrons conjugated into the benzene ring, which reduces their capacity to form hydrogen bonds. As a result, the boiling points of these molecules are typically more than those of other molecules.
- The melting and boiling values of aromatic amines are greater than those of aliphatic amine compounds. It rises in percentage to the molecular mass.
- In comparison to n-hexyl amines, aniline is more water-soluble. Aromatic amines are only marginally soluble in water but dissolve entirely in organic solvents.
- Amines have a higher boiling point than analogous phosphine. However, their boiling point is lower than related alcohols. The characteristics of primary and secondary amines are influenced by hydrogen bonding. Under ordinary conditions, the associated methylamines and ethyl amines are gasses since methyl alcohol and ethyl alcohol are liquids. Similar to liquid amines, gaseous amines have a unique “fishy” odour, whereas liquid amines have a distinct ammonia odour.
- Aromatic amines have a foul stench. It’s a clear liquid or solid chemical with no colour. This oily liquid aniline is easily manufactured. Aromatic amines also become brown when exposed to light or air.
What Exactly are Aliphatic Amines?
Only alkyl groups and hydrogen atoms are directly linked to nitrogen in aliphatic amines. The number of alkyl groups in a compound might range from one to three. Primary amines, secondary amines, and tertiary amines are classified according to the number of connected alkyl groups. Only one alkyl group—1o—represents primary amines. Two alkyl groups—2o—represent secondary amines, and only three alkyl groups—3o—represent tertiary amines. Aliphatic amines have weak bases similar to ammonia.
The basicity rises because alkyl groups replace the hydrogen groups on the nitrogen atom. Primary and secondary amines are greater than tertiary amines. Heterocyclic amines are shaped, while nitrogen is one of the atoms in a ring. Aliphatic heterocyclic amines include piperidine and pyrrolidine.
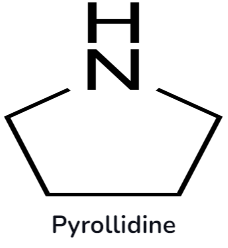
Physical Properties of Aliphatic Amine
Here are some features of aliphatic amines:
- Aliphatic amines have a low boiling point.
- With increasing molecular mass, solubility decreases. The creation of H-bonds in organic solvents such as amines, benzene, and ether makes aliphatic amines water-soluble.
- Aliphatic amines have a distinct fishy smell. C1-C3 amine is a colourless gas at room temperature, but higher amines are liquids or solids.
Conclusion
The NH2 group is usually connected to a C6H5 group, an electron-withdrawing group, in aromatic amines. As a result, the lone pairs of electrons at N drops. Aliphatic amine compounds are, therefore, greater than aromatic amines. Chemical, pharmaceutical, rubber, plastics, textile, cosmetics, and metal industries use aliphatic amines. Intermediates, solvents, rubber accelerators, catalysts, emulsifiers, synthetic cutting fluids, corrosion inhibitors, and flotation agents are all examples of these compounds.
They are used in making rubber and reducing oils. They are also used as intermediates in azo dye manufacturing, and as insecticides. Aromatic amines are linked with bladder cancer.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






