Condensation, also known as dehydration synthesis, involves joining two molecules to form a larger molecule while simultaneously releasing the smaller molecule involved in the reaction. Water, methanol, hydrogen chloride, and acetic acid are some of the tiny molecules that are routinely lost during a biological response, but water is the most frequently lost.
Carbonyl and carboxyl ester moieties are the most significant activating groups in chemical synthesis. The extraction of a proton from an α-carbon atom of a carbonyl group with base results in the equivalent α-carbanion, which is resonance stabilised by the presence of an enolate anion in the reaction. Base catalysed carbonyl compound reactions use these enolate ions.
Among these base-catalysed reactions, the Stobbe condensation process involves the condensation of carbanion, and ketone is an efficient way to form carbon-carbon bonds.
Stobbe Condensation
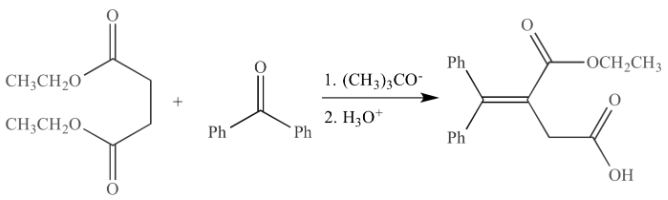
For the first time, in 1893, Stobbe proved that when sodium ethoxide was used on an acetone-diethyl succinate mixture, the expected condensation to produce the beta-diketo molecule did not occur. The Stobbe condensation occurs when aldehydes or ketones combine with the esters of succinic acid to generate alkylidene succinic acid or isomers synthesised by a tautomeric transfer of hydrogen. The metal alkoxide is essential for every mole of carbonyl molecule and ester used, with the principal product being salt of half-ester as the result of the reaction.
Alkylidene succinic acid is formed through the Stobbe condensation process, in which an aldehyde or ketone reacts with a succinic ester in the presence of a basic catalyst such as sodium hydroxide or potassium tertiary butoxide.
A variety of substituted carbonyl compounds, such as aldehydes, aromatics, and aliphatic ketones, were condensed in anhydrous form, as was an active methylene molecule, dimethyl succinate. The reaction can be carried out at room temperature in a dry agate mortar, eliminating the dangers of employing any solvent.
The reaction requires succinic ester; however, the carbonyl component can be varied. The following carbonyl compounds may be used:
- Aldehydes that are aliphatic and-unsaturated
- b Ketones include aliphatic, aromatic, alicyclic, among other things.
Key Features
α – ω Diester ester groups on the first and last carbons can only participate in this reaction.
- The reaction proceeds via a lactone intermediate, catalysed by a base in the following step.
- Elimination results in nearly irreversible ring-opening, resulting in the formation of salts of unsaturated esters. Because of the lactone intermediate discovery, the mechanism can now be more solidly established. Esters containing only one – hydrogen are incapable of undergoing irreversible ring formation.
- The final phase is the opening, and this is what truly drives the forward reaction. Only lactone intermediates have been extracted from these esters.
- If only one – carbon contains two – hydrogens, the reaction can continue to the final step.
Mechanism
The mechanism comprises three phases: the first is the treatment of succinate with a base to produce the following resonance stabilised hybrid. To begin, a carbanion is produced.
- Step 1 comprises carbanion and ketone condensation.
- Step 2 demonstrates a rearrangement and intramolecular cyclisation to give a lactone-ester.
Step 3 entails the ring-opening taking place to give the unsaturated ester acid.
The carbanion is formed due to the deprotonation of the diethyl succinate by the base. In this way, the carbonyl component is nucleophilically enhanced.
The formed oxygen-anion acts as a nucleophile, attacking the nearby carbon atom. As a result, cyclic molecules are built up over time.
The ethanol is subsequently separated from the lactone ring, opening the ring. Finally, the ring is opened through base catalysis.
The intermediate product is subsequently protonated, resulting in the formation of the α, β-unsaturated ester.
Stobbe condensation is a reaction that occurs when metal alkoxide is used as a substrate in refluxing alcohol, most notably butanol. The reaction calls for a dry solid form of potassium tertiary butoxide. The advantages are that the components are affordable and readily available, the quick reaction time, the great yields, and the environmental acceptable reaction conditions.
At room temperature, the solvent-free condensation of substitution ketones and aldehydes with dimethyl succinate happened smoothly, yielding substituted acid esters. Acid esters are formed without heat energy, demonstrating that the reaction is viable under environmentally benign reaction circumstances. Additionally, this strategy increased yields and decreased reaction time.
Application
Condensation is often used to substitute a propionic acid residual for the carbonyl group in aromatic ketones. When combined with the Friedel-Crafts reaction, this condensation can yield naphthols, indones, and tetralones. Additionally, γ-keto acids can be synthesised. The high yields that result from the practically irreversible ring-opening are another benefit. Additionally, the carbon chain can be extended by three carbon atoms simultaneously instead of the Knoevenagel reaction, which adds just two carbon atoms.
Conclusion
The Stobbe condensation, known commonly as the succinic esters condensation, is an organic chemistry reaction. In 1893, Hans Stobbe discovered this synthesis. When esters condense with aldehydes or ketones in a strong base, this is referred to as a specific case of condensation. Stobbe reaction involves the condensation of carbanion and ketone and a succinic ester in the presence of a catalyst such as sodium hydroxide or potassium tertiary butoxide, resulting in the formation of alkylidene succinic acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






