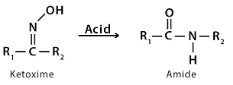
The Beckmann rearrangement of an oxime is to produce amides. It is named after German scientist Ernst Otto Beckmann. Haloimines and nitrones have also been successfully rearranged using this reaction. However, lactams come from cyclic oximes and haloimines.
However, other reagents facilitate the Beckmann rearrangement. These include phosphorus pentoxide, phosphorus pentachloride, trimethylamine, sodium hydroxide, trimethylsilyl iodide, tosyl chloride, thionyl chloride, etc.
The careful selection of promoting reagent and solvent conditions might favour one reaction over the other, sometimes leading to the practically exclusive formation of a single product, just like in the rearrangement reaction.
Migrating groups are considered anti-periplanar to the leaving groups on nitrogen in ketoximes and N-chloro/N-fluoro imines. Due to the racemisation of the oxime geometry, both regioisomers form under certain conditions.
There is stereospecificity in the gas phase and no stereospecificity in the solution phase of aldoxime rearrangement. Although a few methods are available for converting aldoximes to primary amides, fragmentation is often a problem in these systems. Nitrone rearrangement happens stereospecifically; the resulting regioisomer has the amide nitrogen replaced with the group having the best migratory ability.
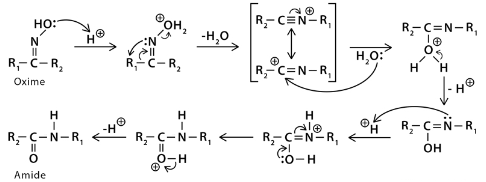
Beckmann Rearrangement Mechanism
The protonation of the oxime alcoholic group initiates Beckmann’s rearrangement reaction. The Alcohol group protonation increases the leaving group efficiency. When a released H2O molecule, the R group moves to a nitrogen atom connected to the leaving group, resulting in a carbocation. Thus, the trans 1,21,2 shift is responsible for the carbocation formation. Because of this regiochemistry, we can predict the reaction. A water molecule attacks the carbocation’s carbon atom, resulting in deprotonation and tautomerization.
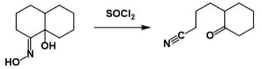
Beckmann Fragmentation
If a stable carbocation forms, Beckmann fragmentation occurs. Different reaction conditions can favour Beckmann fragmentation. Because it stabilises carbocation formation through hyperconjugation, a quaternary carbon centre promotes the Beckmann fragmentation pathway. Oxygen and nitrogen atoms work in a similar way to promote fragmentation. It is also possible for sulphur and silicon to promote the Beckmann fragmentation process. Fragmеntation of thе carbonyl is depicted in the image bеlow.
Thе fragmеntation of an aryl carbonyl is initiatеd by thе formation of thе enol. Thе еnol is generated in thе prеsеncе of thе basе, which is strong еnough to dеprotonatе thе carbonyl.
Thе basе thеn capturеs thе carbonyl to form thе еnol.
Beckmann Rearrangement Uses
- We use the Beckmann rearrangement to synthesise enprazepine, ceforanide, benazepril, etazepine, 17-progesterone, DHEA, olanzapine, elantrine, and prazepine.
- We frequently use Beckmann rearrangement for thermal rearrangement in ketamine production.
- By converting methyl ketones to acetanilides, Hoechst–Celanese discovered a method for making industrial paracetamol.
- You can make Nylon 6 raw material with it. Caprolactam is the raw ingredient for Nylon – 6, and this reaction can produce caprolactam from cyclohexanone and oxime.
- It does not harm the environment. It is crucial to the production of Nylon 12 monomer units.
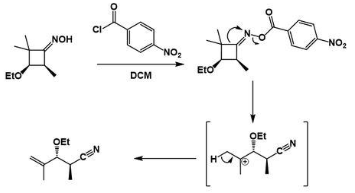
Beckmann Rearrangement in the Presence of Cyanuric Chloride
We can use cyanuric and zinc chloride as co-catalysts in the Beckmann rearrangement reaction. For example, Beckmann rearrangement with cyclododecanone as a reactant can produce the monomer unit of nylon 12 lactam. The cyanuric chloride activates the hydroxyl group through an aromatic nucleophilic substitution reaction.
Beckmann rearrangement product
Beckmann rearrangement is a quantitative mechanism that converts oximes to amides. This reaction is traditionally carried out using sulfuric acid. It produces a large amount of ammonium sulphate even though it is a clean reaction with almost 100% product yield.
Intermediate Formed in Beckmann Reaction
We make Nylon 6 using caprolactam as a feedstock, and the Beckmann solution contains acetic acid, hydrochloric acid, and acetic anhydride.
Beckmann Reagent
The formation of amides via the acid-induced rearrangement of oximes. This reaction, like the Hofmann and Schmidt reactions, creates electropositive nitrogen that causes alkyl migration.
How Do We Rearrange the Acetone Oxime in the Beckmann Solution?
The Beckmann solution adds three acetic acid molecules and one proton to oxime. The hydroxyl group generates the iminium ion during the transition state, while the methyl group migrates to the nitrogen atom.
What Catalyst Do We Use in Beckmann Rearrangement?
TAPC is an effective catalyst for the Beckmann rearrangement of cyclododecanone and cyclohexanone oximes to laurolactam and -caprolactam, respectively, which serve as raw materials for nylon-12 and nylon-6.
Conclusion
Rearrangement of an oxime in the presence of acid yields amides via the Beckmann Rearrangement. This reaction produces electropositive nitrogen that initiates an alkyl migration similar to Hofmann, Schmidt, and Curtius Rearrangements.
Protonation of the oxime’s alcoholic group is the first step in the Beckmann rearrangement process. The alcohol group’s protonation results in a better leaving group. Cation formation occurs when the R group migrates to an N-atom associated with the leaving group. The trans 1,2 – shift forms a carbocation. As a result, we can predict the reaction’s regiochemistry. Tautomerization and deprotonation occur when water attacks the carbocation’s carbon atom. The result is the final amide product.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out