Gas is described as a homogeneous fluid with low density and low viscosity. The volume of the gas is considered equal to the volume of the vessel. Gas is classified into two categories: Ideal gas and non-ideal gas (often known as natural gas). Gas molecule behaviour is determined by the qualities and laws followed by the molecules of gas. The arrangement of the molecules in a gas differs substantially from that of molecules in solids and liquids.
The behaviour of gas molecules is determined by five characteristics and five gas laws: Boyle’s law, Avogadro’s law, Ideal gas law, Charles’s law, and Gay-Lussac’s law. We will briefly discuss the following laws in the below notes.
What Is The Common Behaviour Of Gasses?
Liquid, gas, solid, Bose-Einstein condensate, and plasma are the five primary states of matter. Gases follow a set of rules referred to as gas Laws. These rules describe the behaviour of gasses, and the values and relationships of pressure, volume, and temperature.
What Is Gas Law?
In the modern periodic table of the different elements, the group consisting of the inert or permanent gasses, which are very unreactive, is found. Their features are very similar to an ideal gas, and as a result, their behaviour is quite similar to that of an ideal gas. The following principles controlling the behaviour of gasses were derived based on various studies employing inert gasses:
Boyle’s lLaw
Boyle’s law says that the volume (V) of the mass of given gas is inversely proportional to the pressure (p) of the gas if the temperature remains constant. Hence, we can conclude that-
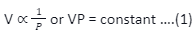
Here, the constant denotes the proportionality constant. The constant value depends upon the temperature, nature, and mass of the gas. Let’s assume that P1 & V1 are the initial and final pressure and volume, respectively, of any gas, and P2 & V2 are another value set,
Hence, we can say that-
P₁V₁ = constant …(2)
P2V2 = constant …(3)
As we have read above, the gas’s mass, nature, and temperature remain the same throughout the process; we conclude that the equation (2) & (3) denotes the same quantity. So-
P₁V₁ = P2V2
Charles’ Law
The volume & temperature of an ideal gas has a similar relationship, known as Charle’s law. This law asserts that the volume (V) of a given amount of gas is directly proportional to the absolute temperature at constant pressure (T).
V/T = constant, if V is the volume and T is the temperature of a gas at a constant pressure. Using the same method as before, we can write-
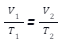
Regnault’s Law, or Gay Lussacs’ Law
Gay Lussacs’ Law says that the pressure (P) of mass of a gas is directly proportional to absolute temperature (T) at constant volume (V). We can write-
P ∝ T or P/T = constant
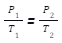
What Do You Mean By The Compressibility Of Gasses?
Compressibility denotes how much the volume of an object shrinks when a certain pressure is applied. When the pressure is applied to the liquid or solid, the volume of the objects does not change too much. As per the kinetic-molecular theory, gasses are more compressible than solids or liquids, because the volume of the gas is made up of extensive quantities of space among the particles of the gas.
What Are The Factors Affecting Gas Pressure?
The various factors affecting gas pressure are as follows:-
- Temperature (T)
- Pressure (P)
- Volume (V)
- Quantity (n)
The factors listed above are interconnected and are listed as follows:
- The gas’s volume expands with the rise in temperature because of the expansion of molecules
- The temperature of the gas molecule decreases with the contraction of the gas molecule
- The temperature rises due to the expansion in the gas
- The temperature falls due to the contraction in the gas
Conclusion
In the above chapter, we have read about gasses’ common behaviour, such as the compressibility of gasses and factors affecting gas pressure. Gasses are the simplest among the other elements, such as solid, liquid, and plasma. Scientists have conducted several experiments over the past 4 centuries to understand the general behaviour of gasses better. They discovered that the physical state of a gas is determined by four variables: Volume (V), temperature (T), quantity (A) and pressure (P). The gas laws, which reflect our current knowledge of how gasses behave on a macroscopic level, are the relations between these variables. However, the connections underlying the gas laws were not clear at first—they were discovered over many years by numerous scientists investigating and verifying their theories about gasses.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



