The phrase “thermodynamic” refers to an area of physics concerned with heat, work, and energy forms. The term “equilibrium” refers to the condition of balance within a system as well as between the system and its environment.
What is Thermodynamic Equilibrium?
Talking about the definition of thermal equilibrium – when no spontaneous change in any macroscopic attribute is detected, and the system is isolated from its surroundings, the system is considered to be in thermodynamic equilibrium.
As you may be aware, temperature is a measurement of how hot or cold a body is compared to a reference item. Two essential ideas are necessary when discussing temperature changes: thermal contact and thermal equilibrium. If two items can alter each other’s temperature, they are in thermal contact. Thermal equilibrium is attained when two items in thermal contact no longer affect each other’s temperature. If, for example, a bottle of milk from the refrigerator is positioned on the counter, the two things are in thermal contact. After so many hours, their temperatures are the same, suggesting that they are in thermal equilibrium.
Types of Thermodynamic Equilibrium:
After discussing what is thermal equilibrium, there is a total 3 thermodynamic equilibriums which are:
- Chemical Equilibrium
- Mechanical Equilibrium
- Thermal Equilibrium
1. Chemical Equilibrium
When no chemical reactions occur within the system or between the system and its surroundings, the system is considered to be in chemical equilibrium. The chemical composition will be consistent throughout the system, and the system’s chemical balance will not be disturbed. This is a reaction that can be reversed.
Example:
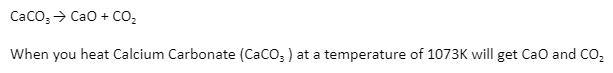
2. Mechanical Equilibrium
The system is said to be in mechanical equilibrium when there are no unbalanced forces inside it or between it and its environment.
The pressure in the system is constant throughout and does not change over time.
Example:
Treadmill: This is a gym machine on which we run but do not move ahead since the force pushing you forward is the same force pushing you backwards. As a result, one of the best instances of mechanical equilibrium may be seen here.
3. Thermal Equilibrium
When there is no temperature difference and the temperature remains constant throughout a system, it is said to be in thermal equilibrium.
Example:
The temperature of a hot cup of tea is higher than the ambient temperature; hence it is not in thermal equilibrium.
However, if you leave it out in the open for a while, the temperature begins to radiate into the environment, and the ambient temperature and the temperature of a cup of tea remain the same at that point; we may say it is in thermal equilibrium.
What are the different types of thermal equilibrium used in thermodynamics?
In terms of thermal equilibrium, it is the relationship between two thermally connected items.
It is an example of equilibrium between two bodies, relating to the transmission across a selectively permeable partition of matter or work; it is referred to as a diathermal link after considering the definition of thermal equilibrium. According to Lieb and Yngvason, the primary meaning of the thermal equilibrium link is that it is reflexive and symmetric. The basic definition does not specify whether it is transitive or not. After studying the semantics of the statement, they propose a significant physical postulate, which they call the “zeroth law of thermodynamics”. It states that thermal equilibrium is a transitive connection. According to them, isotherms are the equivalence classes of created systems.
An isolated body’s internal thermal equilibrium
The state of a body, when it is isolated, is referred to as the “thermal equilibrium of a body.” The background is that it receives no heat and is allowed to return to its native state for an infinite amount of time. It has reached thermal equilibrium when it has completely settled to the point where macroscopic change is no longer visible. It doesn’t mean it’s in any other kind of internal equilibrium. Glass is an example of a body that achieves internal temperature equilibrium but not internal chemical equilibrium.
Conclusion
That’s a wrap to the definition of thermal equilibrium and what are the different types of thermal equilibrium used in thermodynamics!
The concept of equilibrium, on the surface, gives the message that something “balances out.” Equilibrium is a situation in which all driving forces or gradients have vanished, and everything remains the same. Because there are no driving factors causing anything to change, a system in equilibrium maintains its existing condition. Thermal equilibrium is achieved when two materials have the same temperature. There is no heat exchange since there are no thermal gradients.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




