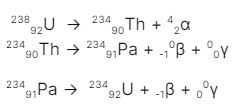Gamma rays were first observed during radioactive decay. In 1899, Rutherford first differentiated between this and the other two rays. Initially, he considered this ray as a fast beta particle. But later, with the help of a magnetic field test, when it was established that it has no charged particle, then that old idea was rejected. In 1914, it was proved that these rays are nothing but EM waves with the help of a reflection test on the crystal surface.
Gamma rays
As mentioned above, this ray is produced in different reactions, but we shall focus only on the radioactive decay reactions.
During a radioactive decay reaction, when alpha or beta or both occur in a nucleus of an atom, it gets excited. Just like an electron returns to its lower energy state by emitting photons (not a gamma ray photon), in the same way, an excited “daughter” nucleus also emits photons of the lowest wavelengths for gaining stability. Such reactions are one of the important sources of gamma rays. An example of such reactions is:
23491Pa(excited) —> 23491Pa + γ (pa: protactinium)
6027Co —> 6028Ni* + e– + v–e + E
2860Ni* —> γ + E
There is another type of reaction where gamma rays are produced, which is called electron capture. This is the opposite process of beta production. An example of one such reaction is:
20180Hg + 0-1e —> 20179Au + γ
Some other sources of gamma rays:
Gamma ray bursts: This is the most powerful gamma explosion that happened in the universe. It released more energy than the sun can in its entire lifetime.
Supernova: During a supernova explosion, massive amounts of gamma rays are produced.
Lightning also produces gamma rays in the earth’s atmosphere.
Properties of gamma rays:
Following are the properties of gamma rays:
Gamma rays contain ~10-12 m frequency electromagnetic waves.
Gamma radiation is an exceptionally incredible photon with an extremely short frequency (0.0005 to 0.1 nm).
Gamma discharges frequently go with alpha and beta emanations as an energised core falls into a lower and more steady energy condition.
Gamma rays are high-recurrence electromagnetic waves without mass and charge. This radioactive outflow has the minimal force of ionisation.
These are transmitted because of the change from a higher energy state to bring down the energy condition of the energised core.
Gamma rays transmit energy when an electron moves to a lower energy state.
The entrancing power is the most noteworthy for gamma rays. They have minimal ability to ionise any material. However, they are the riskiest.
Gamma rays have the most extraordinary infiltrating power, and a couple of centimetres of lead sheet or a couple of metres of cement can stop them. In specific cases, they might even go through them.
Basic terms used in gamma rays:
Ionising power/Ionisation potential:
Ionising power of a radiation is the capacity of that radiation to ionise other molecules when it is passed through it. This ionisation happens because when a radiation passes through a medium, the atoms of that medium lose electrons and get ionised. The more this property will be, the more the radiation will have ionising power. For gamma rays, the ionising power is very low.
Penetration effect:
There is another property called the penetration effect. Radiation can penetrate the human body and go through it. This is the penetration effect. The more that capacity will be, the more penetrating will that ray be. Gamma rays have a high penetrating power.
In general, the greater mass present, the greater the ionising power and lower the penetration power.
Comparison of alpha, beta, and gamma rays
Property | Alpha | Beta | Gamma |
Nature | Positively charged helium particle | Negatively charged particle | Em waves |
Charge | Positively charged (2 units) | Negative charged (1 unit) | No charge |
mass | Mass of a helium atom | Mass of electron | Massless |
Ionisation potential | Highest | Moderate | Lowest |
Penetration power | Lowest | Moderate | Highest |
Range (approx) | 10 cm in air | Few metres | Several metres |
From the above discussion, it is quite clear how gamma rays originated and how they are different from other radioactive rays.
Usages of gamma rays:
One of the most important uses of gamma rays is in the treatment of cancer. Cancer cells are killed using radiotherapy, and it is done by gamma rays.
The sterilisation process of medical equipment
To check weak points in a large oil pipeline
Used in nuclear reactors
Used by scientists to detect astronomical objects
Harmful effects of gamma rays:
Mild radiation sickness: Because of small doses of gamma rays for a prolonged time, fatigue and weakness can occur.
Severe radiation sickness: Problems like skin burn, hair loss, headache, poor healing, etc. can occur.
Cancer: Being exposed to gamma rays for a long time can cause cancer (blood, hepatic, etc).
Conclusion:
Gamma rays are one of the most important types of EM waves. Due to its very high value of penetration power, an object that radiates these rays is conserved in lead-coated boxes. In physics and astronomy, these rays have immense uses. For example, with the help of gamma ray waves, spectroscopy scientists measure the amount of gamma ray radiated from a sample, and by measuring this amount, different elements can be identified. This concept is applied by researchers to know the geochemistry and composition of other planets.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out