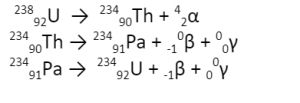There are three main types of rays based on the particles emitted as radiation: Alpha, Beta and Gamma Rays. Alpha radiation of alpha rays is a heavy, short-range particle created when a helium nucleus is expelled. Radium, Thorium, uranium and radon are some alpha emitters. Beta radiation or beta rays are short-range, light particles created on the expulsion of an electron. Strontium-90, tritium, carbon-14 and sulphur-35 are some beta emitters. Gamma rays are highly penetrating electromagnetic radiation. They are commonly referred to as “penetrating” radiation since they can easily permeate most things.
Alpha rays
Alpha particles are bi-positive helium ions (4He2) denoted by α. Therefore, when a radioactive element loses an alpha particle, its atomic number reduces by two while the mass decreases by 4. For example:
238U92 —-> 4He2 + 234Th90
Uranium has a mass number of 238 and an atomic number of 92. When it emits an alpha particle due to its unstable nuclei, it loses two protons and two neutrons to form Thorium (234 is the mass number and 90 is the atomic number). Thorium, another unstable nucleus, loses an alpha particle to form radium.
234Th90 —–> 4He2 + 230Ra88
The Properties Of Alpha Rays
Alpha rays are the radiation that is positively charged.
The speed of alpha rays goes from 5% to 7% of the light speed.
Despite their high ionisation power, these beams are not extremely unsafe. These are, indeed, the most un-risky of the three beams as long as they are not breathed in.
A couple of centimetres of air can prevent alpha rays from infiltrating through the skin.
Particles with alpha rays have minimal ability to enter. They can’t travel exceptionally far in the air and they can’t traverse a piece of paper.
Beta rays
Beta particles are formed when a neutron changes to a proton, unlike alpha particles. Neutrons are electrically neutral but have a mass. On the other hand, Protons are positively charged particles with a mass equivalent to that of neutrons. Therefore, in ideal conditions, when neutrons change to protons, an additional positive charge will be created, which violates the law of conservation of charges.
This is why to balance the transformation; it is considered that a neutron disintegrates into two particles- a proton with a positive charge and similar mass and a negatively charged electron with no mass. This electron is termed the beta particle. It is denoted by 0e-1.
234Th90 —–> 0e-1 + 234Pa91
Since the beta particle doesn’t have any mass, the mass number of Thorium and protactinium remains the same. However, as a new proton is created, the atomic number of protactinium has increased by 1.
The Properties Of Beta Particles
Beta particles are the fast electrons sent from the centre of an atom.
But the beta-atom and the cathode ray are both fast electrons. In any case, they differ in their beginning stage. Beta-particles are given out from the centre of the atom, while cathode rays radiate out from its orbital electrons.
The speed of Beta particles is in the range of 108 m/s.
Beta particles ionise the gas through which they pass.
Entering power of Beta-particles is more than that of alpha particles.
Beta particles impact a visual plate.
Gamma Rays
Gamma rays, highly energetic electromagnetic radiation generated during any type of reaction, are radiation. Since these radiations have waveforms, they do not have mass or charge. As they are EM waves, their energy is so high that they can easily penetrate through bones, lead walls, etc.
This is why proper measurements must be taken to contain the radiation while harnessing their power. This type of radiation is harmful, so nuclear reactions are artificially triggered within thick concrete blocks as this material can block the gamma radiations.
Properties Of Gamma Rays
Gamma rays contain ~10-12 m frequency electromagnetic waves.
Gamma radiation is an exceptionally incredible photon with an extremely short frequency (0.0005 to 0.1 nm).
Gamma discharges frequently go with alpha and beta emanations as an energised core falls into a lower and more steady energy condition.
Gamma rays are high recurrence electromagnetic waves without mass and charge. This radioactive outflow has the minimal force of ionisation.
These are transmitted because of the change from a higher energy state to bring down the energy condition of the energised core.
Gamma rays transmit energy when an electron moves to a lower energy state by producing a photon to an infrared reach someplace in the bright reach.
The entrancing power is the most noteworthy for gamma rays. They have minimal ability to ionise any material. However, they are the riskiest.
Gamma rays have the most extraordinary infiltrating power, and a couple of centimetres of lead sheet or a couple of metres of cement can stop them. In specific cases, they might even go through them.
Conclusion
Alpha rays are the radiation that is positively charged. An alpha particle is a helium nucleus, indicated by the sign α. The alpha particle is a helium particle that has lost two electrons and has a + 2e charge. Beta rays comprise electrons of high energy. They are less ionising than alpha rays, yet they are more destructive as they infiltrate the skin. With an aluminium sheet, they can be halted. Gamma rays are high recurrence electromagnetic waves without mass and charge. This radioactive outflow has the minimal force of ionisation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out