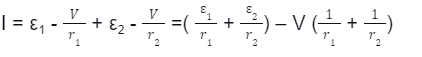A cell is a source of energy in an electrical circuit; two or more combinations of cells form a battery. An electrochemical cell converts the electrical form of energy into the chemical form of power. Alessandro Volta invented the concept of an electric battery; the physical quantity voltage has its SI unit in Volts, named after Alessandro Volta. Cells and batteries have potential applications in electrical appliances and can be connected in a series or parallel combinations. Read the article to learn about a cell, its EMF, internal resistance, a similar variety of cells, and their advantages.
What Is a Cell?
A cell provides the source of electrical energy by converting it from chemical energy when current passes through it due to the two terminals, anode and cathode, wherein the anode is the cell’s negative terminal, and the cathode is the positive terminal of the cell. A combination of two or more cells makes up a battery that converts the stored chemical energy into electrical potential energy, which can be used to energise electrical appliances. A battery consists of an electrolyte, a chemical compound containing ions and thus conducts electricity by a chemical reaction in the electrolyte. The electrons travel between the anode and the cathode, completing the electrical circuit and providing the electric potential to the connected appliance. However, the movement of electrons eventually stops being overused for some time, implying that it’s time to change the batteries.
EMF
The electromotive force that is commonly known as EMF for a cell or a battery is defined as the potential difference between the two terminals of a cell or battery in an open circuit condition, implying that EMF is the potential in the electrical circuit when no current flows in it. It measures the transfer of energy to the charge carriers in the cell. The expression for electromotive force or EMF is e = E/Q, where e is the EMF of the cell, E is the energy, and Q is the charge; it is measured in Volts. Since the potential across the anode is (V+) and the potential across cathode is (V–), hence e = (V+) – (-V–) = (V+) + (V–)
Internal Resistance
Every cell or battery has an internal resistance that hinders/obstructs current flow when connected to an electrical circuit. Due to internal resistance, a voltage drop is observed across a circuit when current flows through it. The anode and the cathode connected to the terminals of a battery filled with an electrolyte provide an internal resistance to the current path and are denoted by ‘r’ and expressed in Ohms or ‘W’. The relation between the potential difference ‘V’, internal resistance ‘r’, current ‘I’, and EMF ‘e’ is written as V = e -Ir.
Cells in Parallel Combination
· The cells or the combination of cells can be connected in series or parallel combinations, each with its unique formula and advantage. In a parallel combination of cells, the voltage remains the same across the entire circuit, and the electrons travel through several parallel branches.
· The arrangement of cells in a parallel combination can be attained in several ways. It is comparatively easier to connect or disconnect new circuit components in a parallel combination without disturbing the circuit.
· Consider a combination of cells connected in parallel combination where the positive terminals are connected and the negative terminals are connected; the total current, I, splits into many parts depending on the number of branches in the circuit.
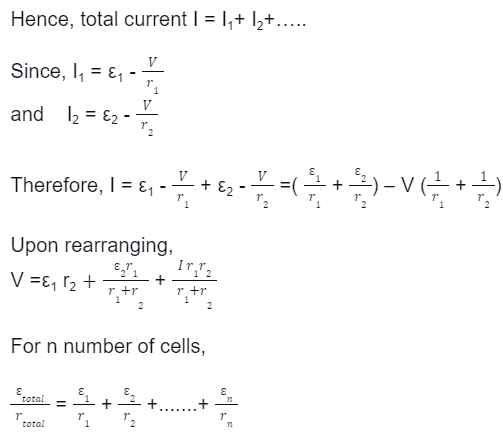
Advantages of Combination
· One of the most important advantages of parallel combination is that if any circuit branch or component gets damaged, it may be easier to replace without disturbing the entire circuit.
· The parallel combination of cells lasts longer than the series combination, and if one appliance is switched on, it will not affect the other appliances.
· Since the voltage remains the same in a parallel combination of cells, each appliance gets a full voltage, and the total resistance in a parallel combination is less than the sum of resistances.
Conclusion
Alessandro Volta invented the concept of batteries; a cell or a battery is a source of electrical energy in a circuit. It converts chemical energy into electrical energy when a current flows through the electrolyte connected between the anode and the cathode. The electromotive force is the potential difference across the cell in an open circuit. Internal resistance is inherently present in the cells that obstruct the flow of current when the cells are connected in series or parallel combinations. The parallel combination of cells has the same voltage throughout and has an advantage over the series combination described in the text.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out