A neutral subatomic particle that is found in every nucleus except simple hydrogen, the neutron is a component of every nucleus. 1.67493 x 10-27 kg is its rest mass, which is slightly higher than the protons but roughly 1,839 times more than the electrons. The nucleus is the atom’s most dense core, where neutrons and protons, known as nucleons, make up 99.9% of the atom’s mass. Quarks, tiny basic particles, are the building blocks of the neutron and the proton, according to twentieth-century developments in high-energy particle physics. The quarks that make up the protons and neutrons in the atom’s nucleus are controlled by the strong force’s residual effect, which holds the nucleus together.
Neutrons
The spin of neutrons, which gives them their magnetic moment, makes them sensitive to magnetic sources in condensed matter and capable of producing images of the magnetic structure. Unlike X-rays, neutrons have the ability to penetrate far into matter. They also interact with nuclei and neutrons can distinguish between light elements such as hydrogen, unlike X-rays.
Neutron drip line
Isotopes contain a constant number of protons, but a variable number of neutrons, which makes them unstable. The neutron drip line is the point at which any extra neutron will not be bonded in the case of the neutron-rich isotopes of each element. When the neutron drip line is crossed, the one- or two-neutron separation energy turns negative, depending on the situation. This means that generating one or two neutrons directly from a nucleus that is beyond the neutron drip line results in a more stable conformation for the nucleus. According to nuclear physics, the location of the neutron drip line coincides with the nuclear nucleus’s limit of existence, which means that nuclei beyond the neutron drip line decay with time scales on the order of 1022 seconds (the time scale of a direct nuclear reaction).
Characteristics of neutron
- They have the ability to penetrate deep into materials because they are electrically neutral.
- The fact that they can operate under severe pressure, temperature, magnetic field and chemical reaction vessel conditions makes them an excellent probe for biological materials and samples.
- They have the ability to demonstrate magnetism under microscopical conditions.
- Having a magnetic dipole moment, neutrons are sensitive to magnetic fields generated by unpaired electrons in materials. As a result, neutrons may be utilized to study the magnetic characteristics of materials at the atomic level. The scattering power of a neutron of an atomic nucleus is also affected by the orientation of the neutron and the spin of the atomic nuclei in a sample, among other factors. As a result, the neutron is an extremely effective tool for detecting nuclear spin order.
- They can detect hydrogen atoms at random because of their sensitivity.
- When it comes to neutrons, the variance in scattering power from one nucleus to another within a sample varies in a manner that is similar to randomness. Because of this, lighter atoms may be seen even when there are heavier atoms present and surrounding atoms can be discriminated from one another. As an additional feature, isotopic substitution (for example, D for H or one nickel isotope for another) can be used to alter the contrast in specific samples, allowing specific structural details to be shown more clearly. The neutron has a high sensitivity to hydrogen atoms, making it an excellent probe for hydrogen storage materials, organic molecular materials and biomolecular samples or polymers, among other things.
- Their angstrom wavelengths are capable of revealing structure.
- Atoms in the solid matter can be separated by as little as 0.1 microns, while neutron wavelengths range from 0.1 microns to 1000 microns. It is for this reason that they are an excellent probe for atomic and molecular structures, whether they be single atomic species or complex biopolymer structures. Neutrons are diffracted by the ordered atoms of a sample in the same way as water waves are diffracted by a barrier.
Conclusion
The neutron is a neutral subatomic particle that is found in every nucleus except simple hydrogen. The nucleus is the atom’s most dense core, where neutrons and protons make up 99.9% of the mass. Neutrons have the ability to penetrate far into the matter and interact with nuclei. Neutrons are electrically neutral particles that can penetrate deep into materials. They can operate under severe pressure, temperature, magnetic field and chemical reaction vessel conditions. The neutron has a high sensitivity to hydrogen atoms, making it an excellent probe for biomolecular samples or polymers.
Conclusion
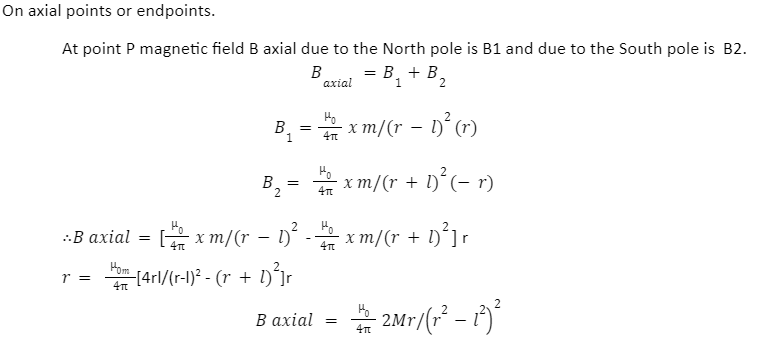
A magnetic dipole is often a tiny magnet with microscopic to subatomic size. It is equal to the flow of electric charge around a loop in the electromagnetic field. The Bohr magneton (equal to 9.27 10-24 ampere–square metre) is a suitable quantity for measuring its magnetic moment. The magnetic dipole moment is the greatest amount of torque induced by magnetic force on a dipole that arises per unit value of the surrounding magnetic field in a vacuum. It can be measured in nanoseconds, and is also known as the magnetic moment.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




