Heat is a form of energy that transfers from one substance to another and brings a temperature change in the final and initial substance. Heat is expressed in units of joules or calories.
Definition of latent heat
Latent heat is the amount of hidden heat required by a substance to undergo a change in its state. During this state change or phase change, the system’s temperature remains constant.
Latent heat is defined as the amount of heat required by the unit mass of a substance to undergo a phase change without undergoing a temperature change. Latent heat is denoted by ‘L’.
History of latent heat
The term latent means hidden or underlying. The concept of latent heat first came into observation by Joseph Black in 1750 while performing an experiment of calorimetry. Black observed that when he heated two same self quantities of water (one from melting of ice and the other, very cold liquid water) using the same method, the temperature at which the liquid of one sample started converting into vapour was lower than the other. He concluded that the phase change took place with the help of the hidden heat or latent heat in the later sample.
Thermodynamic system
The universe consists of two parts – system and surroundings. The part of the universe in which the thermodynamic phenomenon occurs is called the thermodynamic system. The other part, which is surrounding the thermodynamic system, is called the surroundings. A wall is present between the system and the surroundings, known as the boundary. The boundary separates the system from the surroundings.
Calorimetry
The method or experimental technique which is used to measure the heat released or heat absorbed in a chemical reaction is called calorimetry. The results obtained by the calorimetry technique are used to determine whether the reaction is exothermic (the process in which heat is released during a reaction) or endothermic (the process in which heat is absorbed during a reaction).
The calorimetry technique is used to determine the internal energy and enthalpy of a reaction in a thermodynamic system.
Types of latent heat
The latent heat is used to carry out phase change of solid, liquid or gas. The latent heat of various phase transitions can be described as:
- Latent heat of fusion – The process of conversion of a solid substance into its liquid form, for example, the melting of solid ice to form liquid is a fusion process. The amount of heat required to carry out the fusion process of a substance without changing its temperature is called the latent heat of fusion. It is denoted by Lf.
- Latent heat of vapourisation – The process of conversion of a liquid substance into vapour form is called vapourisation. For example, evaporation of water to form water vapour is an example of evaporation. The amount of heat required to carry out the process of evaporation of a substance without changing its temperature is called the latent heat of evaporation. It is denoted by Lv.
- Latent heat of sublimation – The process of conversion of a solid substance into its vapour form is called sublimation. For example, the conversion of solid camphor into vapours is called the sublimation of solid camphor. It is denoted by Ls.
Latent heat: formula
Latent heat is the amount of heat required by the unit mass of a substance to undergo a phase change. The latent heat formula is denoted as –
L = Q ⁄ M
Where,
Q = Amount of heat required to produce a phase change in kJ
M = Mass of substance in kg
L = Latent heat of the specific substance in kJ/kg
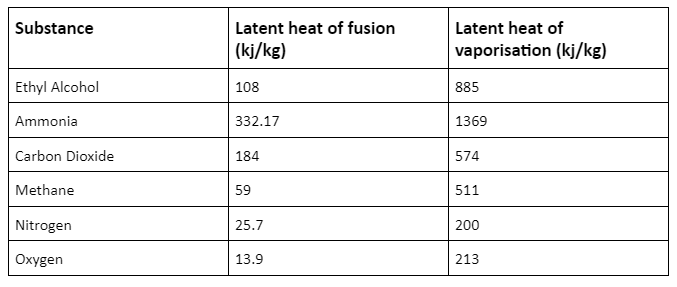
Latent heat of some common gases and fluids
Conclusion
Latent heat is the hidden heat in a reaction, which is involved in carrying out a phase-wise change of a substance without altering its temperature. Latent heat is of mainly two types – latent heat of fusion and latent heat of vaporisation, which are related to fusion and vaporisation phenomena, respectively. Latent heat is defined as the amount of heat required by the unit mass of a substance to change its state without changing its temperature.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




