Introduction
Under this topic we are going to learn about heat engines, types of heat engines and also the efficiency of heat engines. We will also see the different types of thermodynamics processes mentioned in our syllabus. We are also going to deal with the most asked questions about heat engines. After going through this topic your concepts and doubts about heat engines will be very much clear and answered.
So, the topic of heat engines is very much related to the concept of the second law of thermodynamics. Firstly we’ll know what is the second law of thermodynamics?
Second law of thermodynamics
The second law of thermodynamics states that heat transfer occurs spontaneously only from higher to lower temperature bodies.
Key Points
- Many thermodynamic phenomena, allowed to occur by the first law of thermodynamics, never occur in nature.
- Many processes occur spontaneously in one direction only, and the second law of thermodynamics deals with the direction taken by spontaneous processes.
- According to the second law of thermodynamics, it is impossible for any process to have heat transfer from a cooler to a hotter object as its sole result.
Body
What are heat engines?
Heat engine is a device which converts heat into work.
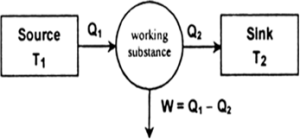
There are three parts of a heat engine:
- Source of high temperature reservoir at temperature T1.
- Sink of low temperature reservoir at temperature T2.
- Working substance.
Types of heat engines
There are two types of heat engines.
- Internal Combustion Engine
- External Combustion Engine
Internal combustion engine.
This burning of fuel known as combustion, occurs inside the system. It is generally known as IC engines. It is a type of heat engine that uses working fuel like petrol and diesel as a source of heat energy. The working process of an IC engine is that it produces work by burning fuel and creating a high-pressure environment. This high pressure is then used to run a turbine or a piston, which converts the heat energy to mechanical energy.
External combustion engine.
The burning of fuel known as combustion, occurs outside the system. It is commonly known as EC engines. In this engine, heat from the burnt fuel is transferred to a secondary liquid, which acts as a fuel for the engine.
The heat engine works in the following manner.
- It consists of a working substance- the system. For example, a mixture of fuel vapour and air in a gasoline or diesel engine or steam in a steam engine are the working substances.
- The working substance goes through a cycle consisting of several processes. In some of these processes, it absorbs a total amount of heat Q1 from an external reservoir at some high temperature T1.
- In some other processes of the cycle, the working substance releases a total amount of heat Q2 to an external reservoir at some lower temperature T2.
- The work done (W) by the system in a cycle is transferred to the environment via some arrangement (e.g. the working substance may be in a cylinder with a moving piston that transfers mechanical energy to the wheels of a cycle via shaft).
-
![working substance-unacadmey]()
The cycle is repeated again and again to get useful work for some purpose. The discipline of thermodynamics has its root in the study of heat engines.
Heat engine efficiency
Before moving to heat engine efficiency; we need to know how we calculate the efficiency of a cycle.
Efficiency Of a Cycle (η):
The efficiency of a cycle is given by .
So,
η= total mechanical work done by the gas in the whole process ÷ heat absorbed by the gas (only +ve)
= area of the P-V cycle ÷ heat injected into the system.
Now, we know what is the efficiency of a cycle. So,
Efficiency of heat engine = work done (W) ÷ heat taken from the source
=(T1-T2)/T1=(Q1-Q2)/Q1
Conclusion
In this topic we have learnt about heat engines. We answered several questions like what are heat engines. We also read about the different types of heat engines. Also, we saw the working of a heat engine and also talked about its efficiency. We have derived the efficiency of a heat engine in the simplest and conceptual manner. Also, we talked about the first law of thermodynamics.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out