Different materials have different capacity to absorb heat to change the temperature in a given mass. Heat capacity is the physical quantity that measures the amount of heat required to change a body’s temperature by a given amount.
heat capacity = Q ⁄ ΔT
The heat capacity is commonly expressed as joules per degree Celsius in terms of the total amount of material used. Heat capacity is an extensive property, which means it is a physical property that measures the size of the physical system. Heat capacities of the materials are usually measured in a variety of calorimeters and by using the third law of thermodynamics. The value of heat capacity is used to calculate the entropies of the various materials.
Heat capacity of gases
The heat capacity of gases is defined as the rise in the temperature of one gram of gases by unit degree by absorbing the heat given to the material. Generally, the heat capacity equation is expressed at constant volume and pressure, which is called the specific heats of gases.
What is the specific heat capacity of gases?:
The units of specific heat are joule per gram per Celsius degree. The specific heat of a material is defined as the rise in temperature by 1 – degree Celsius of a unit mass through giving the heat to the material.
specific heat C = Q ⁄ m .Δ T
The specific heat of gas may vary from 0 to infinity as the gases are compressible. For example, if a gas is compressed, its temperature rises without supplying any heat to it, ie, Q=0. Hence, specific heat
C = Q ⁄ m .Δ T = 0
Also, without any rise in temperature (T=0), if the gas is allowed to freely expand, then
C = Q ⁄ m0 = œ
Therefore, the specific heat of gases is defined by considering any one of the two (either pressure or volume) as constant. Thus, a gas has two specific heats:
- CP, the specific heat at constant pressure, and
- CV, the specific heat at constant volume.
Specific heat at constant volume(CV)
The specific heat capacity of gases at constant volume is an intensive property that measures the amount of heat required by a unit mass of a gas to raise the temperature by 1- degree Celsius, when its volume is kept constant. It is represented by CV
and is given by
CV = (ΔQ ⁄ ΔT) V
The SI unit of the specific heat at constant volume is joule per mass per Celsius degree. This equation is used to find the specific heat capacity of gases at constant volume.
Derivation: The internal energy of a state is a function of state variables like pressure, temperature, volume, etc. Here we have to find the specific heat at constant volume. We consider the volume and temperature as the independent variables.
Thus,
U=f(V,T) ….(i)
Differentiating equation (i) we get
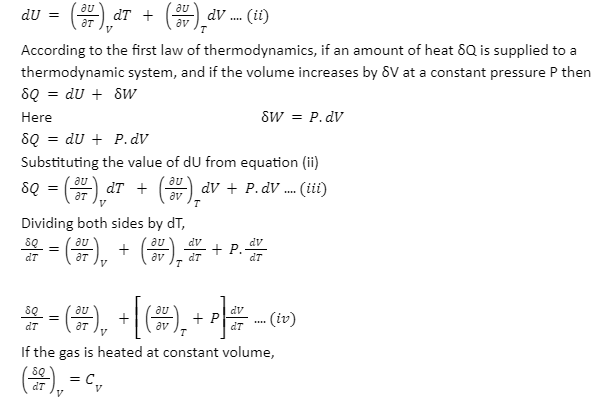

Specific heat at constant pressure(CP):
The specific heat capacity of gases at constant pressure is an intensive property that measures the amount of heat required to raise 1- degree Celsius temperature of a gas’s unit mass under constant pressure. This is represented as CP and is given by
CP = (ΔQ ⁄ ΔT)P
The SI unit of the specific heat at constant pressure is joule per mass per Celsius degree. This equation is used to find the specific heat capacity of gases at constant pressure.
Derivation: The internal energy of a state is a function of state variables like pressure, temperature, volume, etc. Here we have to find the specific heat at constant volume. We consider the pressure and temperature as the independent variables.
Thus,
U=f(P,T) ….(vi)
Differentiating equation (i) we get
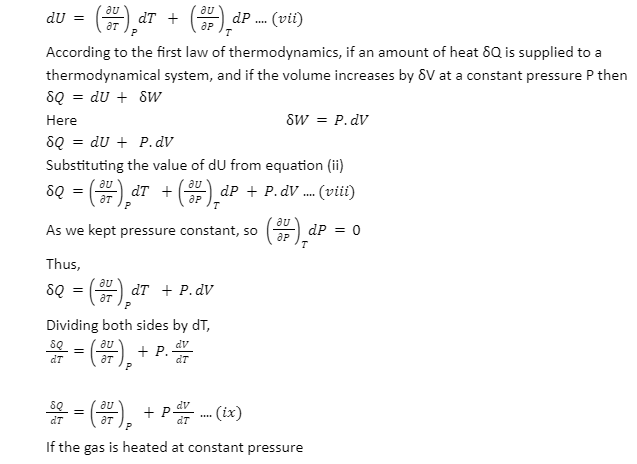
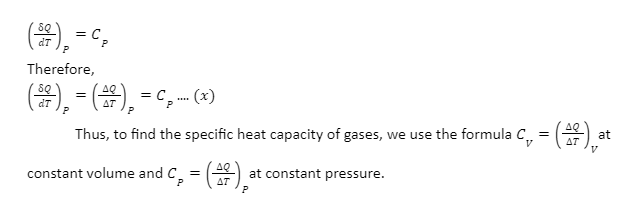
Relation between specific heat at constant pressure and constant volume:
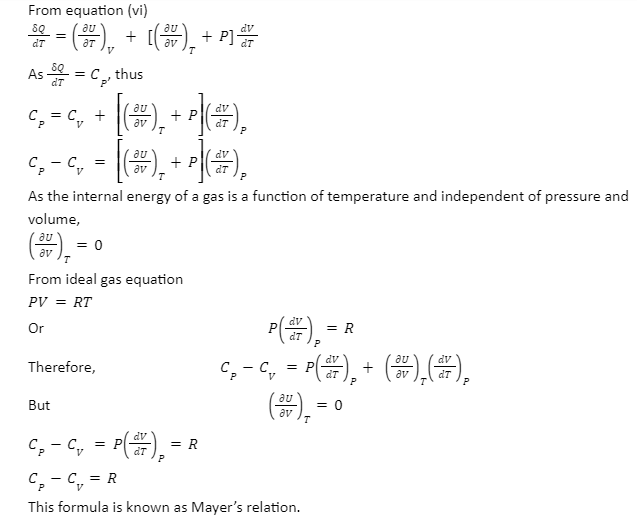
Conclusion
The specific heat of a material is defined as the rise in temperature by 1 – degree of a unit mass through giving the heat to the material.
specific heat C = Q ⁄ m .ΔT
Though this definition may fit the solids and liquids, as the gases are compressible or expandable, the specific heat of gases may vary from 0 to infinity.
The specific heat capacity of gases at constant pressure is an intensive property that measures the amount of heat required to raise 1- Celsius temperature of a gas’s one unit mass under a constant pressure. It is represented by CP and is given by
CP = (ΔQ ⁄ ΔT)P
The specific heat capacity of gases at constant volume is an intensive property that measures the amount of heat required to raise 1- Celsius temperature of a gas’s unit mass, while the volume remains constant. It is represented by CV and is given by
CV = (ΔQ ⁄ ΔT)V.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




