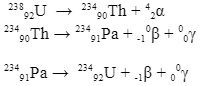Gamma rays were first observed during radioactive decay. In 1899, Rutherford first differentiated between this ray and the other two types. Initially, he considered these rays as fast beta particles. But with the help of a magnetic field test, when it was established that they have no charged particles, then that old idea was rejected. In 1914 with the help of a reflection test on the crystal surface, it was proved that these rays are nothing but EM waves.
Gamma rays
These rays are produced in different reactions, but we shall focus only on radioactive decay reactions.
During a radioactive decay reaction, alpha or beta or both in a nucleus of an atom gets excited. Just like an electron returns to its lower energy state by emitting photons (not a gamma-ray photon), in the same way, an excited “daughter” nucleus also emits photons of the lowest wavelengths to gain stability. Such reactions are one of the important sources of gamma rays. Examples of such reactions are-
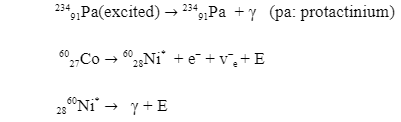
There is another type of reaction where gamma rays are produced, and that is called electron capture. This is the opposite process of beta production. An example of one such reaction is:
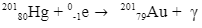
Sources of Gamma Rays
- Gamma-ray bursts: This is the most powerful gamma explosion that happened in the universe. It released more energy than the sun can in its entire lifetime.
- Supernova: During a supernova explosion, massive amounts of gamma rays are produced.
- Lightning also produces gamma rays in the earth’s atmosphere.
Properties of Gamma Rays
Following are the properties of gamma rays:
- Gamma rays contain ~10-12 m frequency electromagnetic waves.
- Gamma radiation is an exceptionally incredible photon with an extremely short frequency (0.0005 to 0.1 nm).
- Gamma discharges frequently go with alpha and beta emanations as an energised core falls into a lower and more steady energy condition.
- Gamma rays are high recurrence electromagnetic waves without mass and charge. This radioactive outflow has the minimal force of ionisation.
- These are transmitted because of the change from a higher energy state to bring down the energy of the energised core.
- Gamma rays transmit energy when an electron moves to a lower energy state by producing a photon to an infrared reach in the bright reach.
- The entrancing power is the most noteworthy for gamma rays. They have minimal ability to ionise any material. However, they are the riskiest.
- Gamma rays have the most extraordinary infiltrating power, and a couple of centimetres of lead sheet or a couple of metres of cement can not stop them. In specific cases, they might even go through them.
Basic terms related to Gamma rays
Ionising power/Ionisation potential
Ionising power of radiation is its capacity to ionise other molecules when it passes through those molecules. When the radiation passes through a medium, the atoms of that medium lose electrons and get ionised. Gamma rays have very low ionising power.
Penetration effect
The ability of radiation to pass through matter, such as the human body, walls, etc., is known as the penetration effect. Gamma rays have a high penetrating power.
In general, the greater the mass present, the greater the ionising power and the lower the penetration power.
Comparison of alpha, beta, and gamma rays
Property | Alpha | Beta | Gamma |
Nature | Positively charged helium particle | Negatively charged particle | EM waves |
Charge | Positively charged (2 units) | Negatively charged (1 unit) | No charge |
Mass | Mass of a helium atom | Mass of an electron | Massless |
Ionisation potential | Highest | Moderate | Lowest |
Penetration power | Lowest | Moderate | Highest |
Range(approx) | 10 cm in air | Few metres | Several metres |
From the above discussion, it is quite clear how gamma rays originated, and how it is different from other radioactive rays.
Gamma Ray Uses
- One of the most important uses of gamma-rays is in cancer treatment. Radiotherapy uses gamma-rays to kill cancer cells.
- Medical equipment is often sterilised using gamma-rays.
- To check weak points in a large oil pipeline.
- They are used in nuclear reactors.
- They are used by scientists to detect astronomical objects.
Harmful effects of gamma-rays
- Mild radiation sickness: Exposure to even a very small dose of gamma for a prolonged time can lead to fatigue and weakness.
- Severe radiation sickness: Problems like skin burn, hair loss, headache and poor healing can occur.
- Cancer: Being exposed to gamma-rays for a longer time can cause cancer (blood, hepatic, etc.).
Conclusion
Gamma rays are one of the most important types of EM waves. Due to its very high value of penetration power, an object that radiates these rays is conserved in lead-coated boxes. In physics and astronomy, these rays have immense uses. For example, with the help of gamma-ray wave spectroscopy, scientists measure the amount of gamma-ray radiated from a sample and by measuring this amount, different elements can be identified. This concept is applied by researchers to know the geochemistry and composition of other planets.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out